Adverse Hemodynamic Conditions Associated with Mechanical Heart Valve Leaflet Immobility
- PMID: 30223603
- PMCID: PMC6165326
- DOI: 10.3390/bioengineering5030074
Adverse Hemodynamic Conditions Associated with Mechanical Heart Valve Leaflet Immobility
Abstract
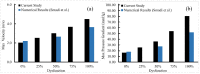
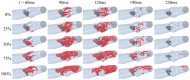
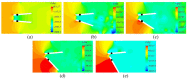
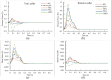
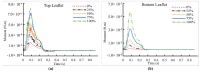
Artificial heart valves may dysfunction, leading to thrombus and/or pannus formations. Computational fluid dynamics is a promising tool for improved understanding of heart valve hemodynamics that quantify detailed flow velocities and turbulent stresses to complement Doppler measurements. This combined information can assist in choosing optimal prosthesis for individual patients, aiding in the development of improved valve designs, and illuminating subtle changes to help guide more timely early intervention of valve dysfunction. In this computational study, flow characteristics around a bileaflet mechanical heart valve were investigated. The study focused on the hemodynamic effects of leaflet immobility, specifically, where one leaflet does not fully open. Results showed that leaflet immobility increased the principal turbulent stresses (up to 400%), and increased forces and moments on both leaflets (up to 600% and 4000%, respectively). These unfavorable conditions elevate the risk of blood cell damage and platelet activation, which are known to cascade to more severe leaflet dysfunction. Leaflet immobility appeared to cause maximal velocity within the lateral orifices. This points to the possible importance of measuring maximal velocity at the lateral orifices by Doppler ultrasound (in addition to the central orifice, which is current practice) to determine accurate pressure gradients as markers of valve dysfunction.
Keywords: adverse hemodynamics; bileaflet mechanical heart valve; blood damage; computational fluid dynamics; platelet activation; transvalvular pressure gradients; turbulent shear stresses.
Conflict of interest statement
No conflict of interest to be declared.
Figures
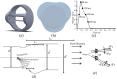

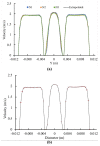
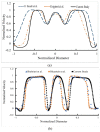
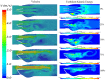

References
-
- WHO Fact sheet: Cardiovascular Diseases (CVDs) [(accessed on 16 September 2018)]; Available online: http://www.who.int/mediacentre/factsheets/fs317/en/
-
- Khalili F., Gamage P.P.T., Mansy H.A. Prediction of Turbulent Shear Stresses through Dysfunctional Bileaflet Mechanical Heart Valves using Computational Fluid Dynamics; Proceedings of the 3rd Thermal and Fluids Engineering Conference (TFEC); Fort Lauderdale, FL, USA. 4–7 March 2018; pp. 1–9.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

