Screening and identification of B-cell epitopes within envelope protein of tembusu virus
- PMID: 30223850
- PMCID: PMC6142368
- DOI: 10.1186/s12985-018-1052-1
Screening and identification of B-cell epitopes within envelope protein of tembusu virus
Abstract
Background: Tembusu virus is a newly emerging flavivirus that caused egg-drop syndrome in ducks in China. TMUV envelope protein is a major structural protein locates at the surface of tembusu virus particle. During tembusu virus infection, envelope protein plays a pivotal role in induction of neutralizing antibody. However, B cell epitopes within envelope protein have not been well studied.
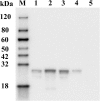
Method: A series of 13 peptides derived from E protein of tembusu virus were synthesized and screened by Dot blot with tembusu virus-positive duck serum. Potential B-cell epitopes were respectively fused with GST tag and expressed in E. coli. The immunogenicity and protective efficiency of epitopes were assessed in ducks.
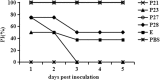
Results: Dot blot assay identified the peptides P21 (amino acids 301-329), P23 (amino acids 369-387), P27 (amino acids 464-471) and P28 (amino acids 482-496) as potential B-cell epitopes within the envelope protein of tembusu virus. Immunization of prokaryotically expressed epitopes elicited specific antibodies in ducks and the specific antibody elicited by P21, P27 and P28 could neutralized tembusu virus. In addition, protective test suggested that P21 and P27 could completely protect immunized ducks from TMUV challenge.
Conclusion: Four potential B cell epiotpes within tembusu virus envelope protein were identified and analyzed in vitro and in vivo. It was demonstrated that two of them (P21 and P27) could elicit neutralizing antibodies in ducks and offer complete protection against tembusu virus challenge. This findings will contribute to the development of epitope vaccine for tembusu virus prevention.
Keywords: B-cell epitope; Envelope protein; Neutralization; Tembusu virus.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Animal Care and Use Committee of Jiangsu Province and the animals were handled by the rules stipulated by the Animal Care and Use Committee of Jiangsu Province.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Design and evaluation of a polytope construct with multiple B and T epitopes against Tembusu virus infection in ducks.Res Vet Sci. 2016 Feb;104:174-80. doi: 10.1016/j.rvsc.2015.09.011. Epub 2015 Sep 18. Res Vet Sci. 2016. PMID: 26850557
-
Identification and immunogenic evaluation of T cell epitopes based on tembusu virus envelope protein in ducks.Virus Res. 2018 Sep 15;257:74-81. doi: 10.1016/j.virusres.2018.09.008. Epub 2018 Sep 18. Virus Res. 2018. PMID: 30240806
-
Identification of a Neutralizing Monoclonal Antibody That Recognizes a Unique Epitope on Domain III of the Envelope Protein of Tembusu Virus.Viruses. 2020 Jun 15;12(6):647. doi: 10.3390/v12060647. Viruses. 2020. PMID: 32549221 Free PMC article.
-
An updated review of avian-origin Tembusu virus: a newly emerging avian Flavivirus.J Gen Virol. 2017 Oct;98(10):2413-2420. doi: 10.1099/jgv.0.000908. Epub 2017 Sep 6. J Gen Virol. 2017. PMID: 28874226 Review.
-
Duck egg drop syndrome virus: an emerging Tembusu-related flavivirus in China.Sci China Life Sci. 2013 Aug;56(8):701-10. doi: 10.1007/s11427-013-4515-z. Epub 2013 Aug 7. Sci China Life Sci. 2013. PMID: 23917842 Review.
Cited by
-
Peptide inhibitors of tembusu virus infection derived from the envelope protein.Vet Microbiol. 2020 Jun;245:108708. doi: 10.1016/j.vetmic.2020.108708. Epub 2020 May 7. Vet Microbiol. 2020. PMID: 32456819 Free PMC article.
-
Shotgun Immunoproteomic Approach for the Discovery of Linear B-Cell Epitopes in Biothreat Agents Francisella tularensis and Burkholderia pseudomallei.Front Immunol. 2021 Sep 29;12:716676. doi: 10.3389/fimmu.2021.716676. eCollection 2021. Front Immunol. 2021. PMID: 34659206 Free PMC article.
-
Immunoinformatics mapping of potential epitopes in SARS-CoV-2 structural proteins.PLoS One. 2021 Nov 15;16(11):e0258645. doi: 10.1371/journal.pone.0258645. eCollection 2021. PLoS One. 2021. PMID: 34780495 Free PMC article.
-
Cytotoxic T-lymphocyte elicited therapeutic vaccine candidate targeting cancer against MAGE-A11 carcinogenic protein.Biosci Rep. 2020 Dec 23;40(12):BSR20202349. doi: 10.1042/BSR20202349. Biosci Rep. 2020. PMID: 33169789 Free PMC article.
-
Predicting Antigenic Peptides from Rocio Virus NS1 Protein for Immunodiagnostic Testing Using Immunoinformatics and Molecular Dynamics Simulation.Int J Mol Sci. 2022 Jul 12;23(14):7681. doi: 10.3390/ijms23147681. Int J Mol Sci. 2022. PMID: 35887029 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

