Cannabidiol enhances morphine antinociception, diminishes NMDA-mediated seizures and reduces stroke damage via the sigma 1 receptor
- PMID: 30223868
- PMCID: PMC6142691
- DOI: 10.1186/s13041-018-0395-2
Cannabidiol enhances morphine antinociception, diminishes NMDA-mediated seizures and reduces stroke damage via the sigma 1 receptor
Abstract
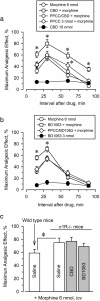
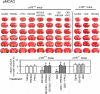
Cannabidiol (CBD), the major non-psychotomimetic compound present in the Cannabis sativa plant, exhibits therapeutic potential for various human diseases, including chronic neurodegenerative diseases, such as Alzheimer's and Parkinson's, ischemic stroke, epilepsy and other convulsive syndromes, neuropsychiatric disorders, neuropathic allodynia and certain types of cancer. CBD does not bind directly to endocannabinoid receptors 1 and 2, and despite research efforts, its specific targets remain to be fully identified. Notably, sigma 1 receptor (σ1R) antagonists inhibit glutamate N-methyl-D-aspartate acid receptor (NMDAR) activity and display positive effects on most of the aforesaid diseases. Thus, we investigated the effects of CBD on three animal models in which NMDAR overactivity plays a critical role: opioid analgesia attenuation, NMDA-induced convulsive syndrome and ischemic stroke. In an in vitro assay, CBD disrupted the regulatory association of σ1R with the NR1 subunit of NMDAR, an effect shared by σ1R antagonists, such as BD1063 and progesterone, and prevented by σ1R agonists, such as 4-IBP, PPCC and PRE084. The in vivo administration of CBD or BD1063 enhanced morphine-evoked supraspinal antinociception, alleviated NMDA-induced convulsive syndrome, and reduced the infarct size caused by permanent unilateral middle cerebral artery occlusion. These positive effects of CBD were reduced by the σ1R agonists PRE084 and PPCC, and absent in σ1R-/- mice. Thus, CBD displays antagonist-like activity toward σ1R to reduce the negative effects of NMDAR overactivity in the abovementioned experimental situations.
Keywords: Acute pain; Cannabidiol; Cannabinoids; Epilepsy; NMDA receptor; Neuropathology; Sigma 1 receptor; Stroke.
Conflict of interest statement
Ethics approval
Protocol from animal studies were approved by Consejo Superior de Investigaciones Científicas (CSIC).
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Griffin G, Atkinson PJ, Showalter VM, Martin BR, Abood ME. Evaluation of cannabinoid receptor agonists and antagonists using the guanosine-5’-O-(3-[35S]thio)-triphosphate binding assay in rat cerebellar membranes. J Pharmacol Exp Ther. 1998;285:553–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

