Evaluating the integration of HIV self-testing into low-resource health systems: study protocol for a cluster-randomized control trial from EQUIP Innovations
- PMID: 30223874
- PMCID: PMC6142354
- DOI: 10.1186/s13063-018-2878-y
Evaluating the integration of HIV self-testing into low-resource health systems: study protocol for a cluster-randomized control trial from EQUIP Innovations
Abstract
Background: Throughout sub-Saharan Africa HIV-testing rates remain low. Barriers to testing, such as inconvenient service hours and long wait times, lack of privacy, and fear of unwanted disclosure, continue to impede service utilization. HIV self-testing (HIVST) is one strategy that addresses these barriers and has been shown to increase use of HIV-testing when distributed through community-based settings. However, the scalability of HIVST is limited because it has yet to be fully integrated into existing health systems and routine care. To address this gap, we designed a study to test the effect of offering HIVST to routine outpatient department (OPD) clients on uptake of HIV-testing as compared to standard of care and optimized standard of care.
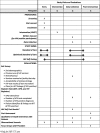
Methods/design: This is a non-blinded, multi-site, cluster-randomized control trial. The health facility is the unit of randomization (cluster). Fifteen facilities were randomized to one of three arms: (1) Standard of care using routine provider-initiated testing and counseling (PITC); (2) Optimized standard of care using optimized PITC defined by additional training, job aids, and monitoring of PITC strategies with OPD providers and support staff; and (3) HIVST defined by HIVST demonstrations for OPD clients, HIVST kit distribution, and private spaces for HIVST kit use and/or interpretation. The primary outcome is the proportion of OPD clients tested for HIV on the day that they accessed OPD services. Secondary outcome measures are the proportion of OPD clients newly identified as HIV-positive and antiretroviral therapy (ART) initiation. Costs and cost-effectiveness will be evaluated. Nested studies will determine the acceptability of facility-based HIVST among OPD clients and health care providers, the presence of adverse events, such as coercion to test or unwanted status disclosure, and a process evaluation to determine feasibility and scale-up of facility-based HIVST for the future.
Discussion: This study protocol tests whether facility-based HIVST can positively contribute to HIV-testing among OPD clients in resource-limited settings. This will be one of the first studies to test the integration of HIVST into facility-based, primary health services in sub-Saharan Africa.
Trial registration: ClinicalTrials.gov, ID: NCT03271307 . Registered on 31 August 2017. Pan African Clinical Trials: PACTR201711002697316 . Registered on 1 November 2017.
Keywords: Cost-effectiveness; HIV; HIV self-testing; HIV testing; Randomized control trial; Sub-Saharan Africa.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol has been reviewed and approved by the Institutional Review Board (IRB) at the University of California, Los Angeles (UCLA) and the National Health Sciences Research Council (NHSRC) in Malawi. Any protocol modifications will be submitted to the IRB Committees for review, and participants will be informed if warranted. Informed consent will be obtained from all study participants prior to enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Economic evaluation of facility-based HIV self-testing among adult outpatients in Malawi.J Int AIDS Soc. 2020 Sep;23(9):e25612. doi: 10.1002/jia2.25612. J Int AIDS Soc. 2020. PMID: 32909387 Free PMC article. Clinical Trial.
-
Barriers and facilitators to facility HIV self-testing in outpatient settings in Malawi: a qualitative study.BMC Public Health. 2021 Dec 2;21(1):2200. doi: 10.1186/s12889-021-12213-6. BMC Public Health. 2021. PMID: 34856958 Free PMC article. Clinical Trial.
-
Effect of index HIV self-testing for sexual partners of clients enrolled in antiretroviral therapy (ART) programs in Malawi: A randomized controlled trial.PLoS Med. 2023 Aug 4;20(8):e1004270. doi: 10.1371/journal.pmed.1004270. eCollection 2023 Aug. PLoS Med. 2023. PMID: 37540649 Free PMC article. Clinical Trial.
-
A systematic review of qualitative evidence on factors enabling and deterring uptake of HIV self-testing in Africa.BMC Public Health. 2019 Oct 15;19(1):1289. doi: 10.1186/s12889-019-7685-1. BMC Public Health. 2019. PMID: 31615461 Free PMC article.
-
The effects of HIV self-testing on the uptake of HIV testing, linkage to antiretroviral treatment and social harms among adults in Africa: A systematic review and meta-analysis.PLoS One. 2021 Jan 27;16(1):e0245498. doi: 10.1371/journal.pone.0245498. eCollection 2021. PLoS One. 2021. PMID: 33503050 Free PMC article.
Cited by
-
Acceptability of index partner HIV self-testing among HIV-positive clients in Malawi: A mixed methods analysis.PLoS One. 2020 Jul 10;15(7):e0235008. doi: 10.1371/journal.pone.0235008. eCollection 2020. PLoS One. 2020. PMID: 32649664 Free PMC article.
-
Verification of HIV Self-Testing Use and Results: A Global Systematic Review.AIDS Patient Care STDS. 2020 Apr;34(4):147-156. doi: 10.1089/apc.2019.0283. AIDS Patient Care STDS. 2020. PMID: 32324482 Free PMC article.
-
Implementation of community and facility-based HIV self-testing under routine conditions in southern Eswatini.Trop Med Int Health. 2020 Jun;25(6):723-731. doi: 10.1111/tmi.13396. Epub 2020 Apr 13. Trop Med Int Health. 2020. PMID: 32219945 Free PMC article.
-
Practical metrics for establishing the health benefits of research to support research prioritisation.BMJ Glob Health. 2020 Aug;5(8):e002152. doi: 10.1136/bmjgh-2019-002152. BMJ Glob Health. 2020. PMID: 32868268 Free PMC article.
-
Lessons learned from implementation of four HIV self-testing (HIVST) distribution models in Zambia: applying the Consolidated Framework for Implementation Research to understand impact of contextual factors on implementation.BMC Infect Dis. 2024 Mar 6;22(Suppl 1):977. doi: 10.1186/s12879-024-09168-5. BMC Infect Dis. 2024. PMID: 38448832 Free PMC article.
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). The Gap Report. Geneva: UNAIDS; 2014.
-
- Meremo A, Mboya B, Ngilangwa D, Dulle R, Tarimo E, Urassa D, Michael E, Gibore N, Mpondo B, McHonde G, et al. Barriers to accessibility and utilization of HIV testing and counseling services in Tanzania: experience from Angaza Zaidi programme. Pan African Medical Journal. 2016;23
-
- Choko AT, Desmond N, Webb EL, Chavula K, Napierala-Mavedzenge S, Gaydos CA, Makombe SD, Chunda T, Squire SB, French N, et al. The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence setting: a cross-sectional feasibility study in Blantyre, Malawi. PLoS Med. 2011;8:e1001102. doi: 10.1371/journal.pmed.1001102. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous