Porcine sapovirus Cowden strain enters LLC-PK cells via clathrin- and cholesterol-dependent endocytosis with the requirement of dynamin II
- PMID: 30223898
- PMCID: PMC6142377
- DOI: 10.1186/s13567-018-0584-0
Porcine sapovirus Cowden strain enters LLC-PK cells via clathrin- and cholesterol-dependent endocytosis with the requirement of dynamin II
Abstract
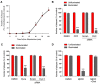
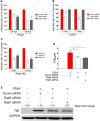
Caliciviruses in the genus Sapovirus are a significant cause of viral gastroenteritis in humans and animals. However, the mechanism of their entry into cells is not well characterized. Here, we determined the entry mechanism of porcine sapovirus (PSaV) strain Cowden into permissive LLC-PK cells. The inhibition of clathrin-mediated endocytosis using chlorpromazine, siRNAs, and a dominant negative (DN) mutant blocked entry and infection of PSaV Cowden strain, confirming a role for clathrin-mediated internalization. Entry and infection were also inhibited by the cholesterol-sequestering drug methyl-β-cyclodextrin and was restored by the addition of soluble cholesterol, indicating that cholesterol also contributes to entry and infection of this strain. Furthermore, the inhibition of dynamin GTPase activity by dynasore, siRNA depletion of dynamin II, or overexpression of a DN mutant of dynamin II reduced the entry and infection, suggesting that dynamin mediates the fission and detachment of clathrin- and cholesterol-pits for entry of this strain. In contrast, the inhibition of caveolae-mediated endocytosis using nystatin, siRNAs, or a DN mutant had no inhibitory effect on entry and infection of this strain. It was further determined that cell entry of PSaV Cowden strain required actin rearrangements for vesicle internalization, endosomal trafficking from early to late endosomes through microtubules, and late endosomal acidification for uncoating. We conclude that PSaV strain Cowden is internalized into LLC-PK cells by clathrin- and cholesterol-mediated endocytosis that requires dynamin II and actin rearrangement, and that the uncoating occurs in the acidified late endosomes after trafficking from the early endosomes through microtubules.
Figures





Similar articles
-
Phosphatidylinositol 3-Kinase/Akt and MEK/ERK Signaling Pathways Facilitate Sapovirus Trafficking and Late Endosomal Acidification for Viral Uncoating in LLC-PK Cells.J Virol. 2018 Nov 27;92(24):e01674-18. doi: 10.1128/JVI.01674-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30282712 Free PMC article.
-
Early Porcine Sapovirus Infection Disrupts Tight Junctions and Uses Occludin as a Coreceptor.J Virol. 2019 Feb 5;93(4):e01773-18. doi: 10.1128/JVI.01773-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463963 Free PMC article.
-
Entry of Classical Swine Fever Virus into PK-15 Cells via a pH-, Dynamin-, and Cholesterol-Dependent, Clathrin-Mediated Endocytic Pathway That Requires Rab5 and Rab7.J Virol. 2016 Sep 29;90(20):9194-208. doi: 10.1128/JVI.00688-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489278 Free PMC article.
-
Taming the Triskelion: Bacterial Manipulation of Clathrin.Microbiol Mol Biol Rev. 2019 Feb 27;83(2):e00058-18. doi: 10.1128/MMBR.00058-18. Print 2019 May 15. Microbiol Mol Biol Rev. 2019. PMID: 30814130 Free PMC article. Review.
-
Disparate Entry of Adenoviruses Dictates Differential Innate Immune Responses on the Ocular Surface.Microorganisms. 2019 Sep 13;7(9):351. doi: 10.3390/microorganisms7090351. Microorganisms. 2019. PMID: 31540200 Free PMC article. Review.
Cited by
-
Sialoglycovirology of Lectins: Sialyl Glycan Binding of Enveloped and Non-enveloped Viruses.Methods Mol Biol. 2020;2132:483-545. doi: 10.1007/978-1-0716-0430-4_47. Methods Mol Biol. 2020. PMID: 32306355 Free PMC article.
-
CD300LF Polymorphisms of Inbred Mouse Strains Confer Resistance to Murine Norovirus Infection in a Cell Type-Dependent Manner.J Virol. 2020 Aug 17;94(17):e00837-20. doi: 10.1128/JVI.00837-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32581099 Free PMC article.
-
CLIC and membrane wound repair pathways enable pandemic norovirus entry and infection.Nat Commun. 2023 Feb 28;14(1):1148. doi: 10.1038/s41467-023-36398-z. Nat Commun. 2023. PMID: 36854760 Free PMC article.
-
Phosphatidylinositol 3-Kinase/Akt and MEK/ERK Signaling Pathways Facilitate Sapovirus Trafficking and Late Endosomal Acidification for Viral Uncoating in LLC-PK Cells.J Virol. 2018 Nov 27;92(24):e01674-18. doi: 10.1128/JVI.01674-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30282712 Free PMC article.
-
Viral Eco-Genomic Tools: Development and Implementation for Aquatic Biomonitoring.Int J Environ Res Public Health. 2022 Jun 23;19(13):7707. doi: 10.3390/ijerph19137707. Int J Environ Res Public Health. 2022. PMID: 35805367 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

