Development of the human prostate
- PMID: 30224091
- PMCID: PMC6234090
- DOI: 10.1016/j.diff.2018.08.005
Development of the human prostate
Abstract
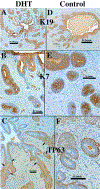
This paper provides a detailed compilation of human prostatic development that includes human fetal prostatic gross anatomy, histology, and ontogeny of selected epithelial and mesenchymal differentiation markers and signaling molecules throughout the stages of human prostatic development: (a) pre-bud urogenital sinus (UGS), (b) emergence of solid prostatic epithelial buds from urogenital sinus epithelium (UGE), (c) bud elongation and branching, (d) canalization of the solid epithelial cords, (e) differentiation of luminal and basal epithelial cells, and (f) secretory cytodifferentiation. Additionally, we describe the use of xenografts to assess the actions of androgens and estrogens on human fetal prostatic development. In this regard, we report a new model of de novo DHT-induction of prostatic development from xenografts of human fetal female urethras, which emphasizes the utility of the xenograft approach for investigation of initiation of human prostatic development. These studies raise the possibility of molecular mechanistic studies on human prostatic development through the use of tissue recombinants composed of mutant mouse UGM combined with human fetal prostatic epithelium. Our compilation of human prostatic developmental processes is likely to advance our understanding of the pathogenesis of benign prostatic hyperplasia and prostate cancer as the neoformation of ductal-acinar architecture during normal development is shared during the pathogenesis of benign prostatic hyperplasia and prostate cancer.
Copyright © 2018 International Society of Differentiation. Published by Elsevier B.V. All rights reserved.
Figures





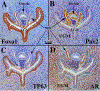

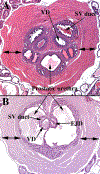



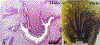


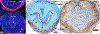





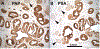
References
-
- Aboseif S, El-Sakka A, Young P, and Cunha G (1999) Mesenchymal reprogramming of adult human epithelial differentiation. Differentiation; research in biological diversity 65:113–118. - PubMed
-
- Adams JY, Leav I, Lau KM, Ho SM, and Pflueger SM (2002) Expression of estrogen receptor beta in the fetal, neonatal, and prepubertal human prostate. The Prostate 52:69–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

