Cox2p of yeast cytochrome oxidase assembles as a stand-alone subunit with the Cox1p and Cox3p modules
- PMID: 30224355
- PMCID: PMC6204888
- DOI: 10.1074/jbc.RA118.004138
Cox2p of yeast cytochrome oxidase assembles as a stand-alone subunit with the Cox1p and Cox3p modules
Abstract
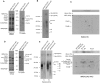
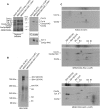
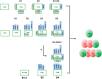
Cytochrome oxidase (COX) is a hetero-oligomeric complex of the mitochondrial inner membrane that reduces molecular oxygen to water, a reaction coupled to proton transfer from the mitochondrial matrix to the intermembrane space. In the yeast Saccharomyces cerevisiae, COX is composed of 11-13 different polypeptide subunits. Here, using pulse labeling of mitochondrial gene products in isolated yeast mitochondria, combined with purification of tagged COX subunits and ancillary factors, we studied the Cox2p assembly intermediates. Analysis of radiolabeled Cox2p obtained in pulldown assays by native gel electrophoresis revealed the existence of several assembly intermediates, the largest of which had an estimated mass of 450-550 kDa. None of the other known subunits of COX were present in these Cox2p intermediates. This was also true for the several ancillary factors having still undefined functions in COX assembly. In agreement with earlier evidence, Cox18p and Cox20p, previously shown to be involved in processing and in membrane insertion of the Cox2p precursor, were found to be associated with the two largest Cox2p intermediates. A small fraction of the Cox2p module contained Sco1p and Coa6p, which have been implicated in metalation of the binuclear copper site on this subunit. Our results indicate that following its insertion into the mitochondrial inner membrane, Cox2p assembles as a stand-alone protein with the compositionally more complex Cox1p and Cox3p modules.
Keywords: Cox2p module; Saccharomyces cerevisiae; biogenesis; cytochrome c oxidase (complex IV); cytochrome oxidase assembly; mitochondria; mitochondrial DNA (mtDNA); mitochondrial respiratory chain complex; yeast genetics.
© 2018 Franco et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

