The regulation of cilium assembly and disassembly in development and disease
- PMID: 30224385
- PMCID: PMC6176931
- DOI: 10.1242/dev.151407
The regulation of cilium assembly and disassembly in development and disease
Abstract
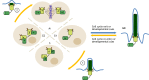
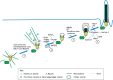
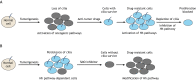
The primary cilium is an antenna-like organelle assembled on most types of quiescent and differentiated mammalian cells. This immotile structure is essential for interpreting extracellular signals that regulate growth, development and homeostasis. As such, ciliary defects produce a spectrum of human diseases, termed ciliopathies, and deregulation of this important organelle also plays key roles during tumor formation and progression. Recent studies have begun to clarify the key mechanisms that regulate ciliary assembly and disassembly in both normal and tumor cells, highlighting new possibilities for therapeutic intervention. Here, we review these exciting new findings, discussing the molecular factors involved in cilium formation and removal, the intrinsic and extrinsic control of cilium assembly and disassembly, and the relevance of these processes to mammalian cell growth and disease.
Keywords: Cancer; Ciliopathies; Cilium assembly; Cilium disassembly; Primary cilia.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


Similar articles
-
Cilium assembly and disassembly.Nat Cell Biol. 2016 Jun 28;18(7):711-7. doi: 10.1038/ncb3370. Nat Cell Biol. 2016. PMID: 27350441 Free PMC article. Review.
-
Functional aspects of primary cilium in signaling, assembly and microenvironment in cancer.J Cell Physiol. 2021 May;236(5):3207-3219. doi: 10.1002/jcp.30117. Epub 2020 Oct 26. J Cell Physiol. 2021. PMID: 33107052 Free PMC article. Review.
-
Moonlighting of mitotic regulators in cilium disassembly.Cell Mol Life Sci. 2021 Jun;78(11):4955-4972. doi: 10.1007/s00018-021-03827-5. Epub 2021 Apr 15. Cell Mol Life Sci. 2021. PMID: 33860332 Free PMC article. Review.
-
Assembling a primary cilium.Curr Opin Cell Biol. 2013 Aug;25(4):506-11. doi: 10.1016/j.ceb.2013.04.011. Epub 2013 Jun 7. Curr Opin Cell Biol. 2013. PMID: 23747070 Free PMC article. Review.
-
Insights into the Regulation of Ciliary Disassembly.Cells. 2021 Nov 1;10(11):2977. doi: 10.3390/cells10112977. Cells. 2021. PMID: 34831200 Free PMC article. Review.
Cited by
-
Loss of polycystins suppresses deciliation via the activation of the centrosomal integrity pathway.Life Sci Alliance. 2020 Jul 10;3(9):e202000750. doi: 10.26508/lsa.202000750. Print 2020 Sep. Life Sci Alliance. 2020. PMID: 32651191 Free PMC article.
-
Composition, organization and mechanisms of the transition zone, a gate for the cilium.EMBO Rep. 2022 Dec 6;23(12):e55420. doi: 10.15252/embr.202255420. Epub 2022 Nov 21. EMBO Rep. 2022. PMID: 36408840 Free PMC article. Review.
-
Gpr63 is a modifier of microcephaly in Ttc21b mouse mutants.PLoS Genet. 2019 Nov 15;15(11):e1008467. doi: 10.1371/journal.pgen.1008467. eCollection 2019 Nov. PLoS Genet. 2019. PMID: 31730647 Free PMC article.
-
Pathogenic RAB34 variants impair primary cilium assembly and cause a novel oral-facial-digital syndrome.Hum Mol Genet. 2023 Sep 5;32(18):2822-2831. doi: 10.1093/hmg/ddad109. Hum Mol Genet. 2023. PMID: 37384395 Free PMC article.
-
FOP Negatively Regulates Ciliogenesis and Promotes Cell Cycle Re-entry by Facilitating Primary Cilia Disassembly.Front Cell Dev Biol. 2020 Nov 12;8:590449. doi: 10.3389/fcell.2020.590449. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33304902 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

