Atrial fibrillation detection using single lead portable electrocardiographic monitoring: a systematic review and meta-analysis
- PMID: 30224404
- PMCID: PMC6144487
- DOI: 10.1136/bmjopen-2018-024178
Atrial fibrillation detection using single lead portable electrocardiographic monitoring: a systematic review and meta-analysis
Abstract
Objectives: Recent technology advances have allowed for heart rhythm monitoring using single-lead ECG monitoring devices, which can be used for early diagnosis of atrial fibrillation (AF). We sought to investigate the AF detection rate using portable ECG devices compared with Holter monitoring.
Setting, participants and outcome measures: We searched the Medline, Embase and Scopus databases (conducted on 8 May 2017) using search terms related to AF screening and included studies with adults aged >18 years using portable ECG devices or Holter monitoring for AF detection. We excluded studies using implantable loop recorders and pacemakers. Using a random-effects model we calculated the overall AF detection rate. Meta-regression analysis was performed to explore potential sources for heterogeneity. Quality of reporting was assessed using the tool developed by Downs and Black.
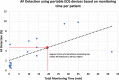
Results: Portable ECG monitoring was used in 18 studies (n=117 436) and Holter monitoring was used in 36 studies (n=8498). The AF detection rate using portable ECG monitoring was 1.7% (95% CI 1.4 to 2.1), with significant heterogeneity between studies (p<0.001). There was a moderate linear relationship between total monitoring time and AF detection rate (r=0.65, p=0.003), and meta-regression identified total monitoring time (p=0.005) and body mass index (p=0.01) as potential contributors to heterogeneity. The detection rate (4.8%, 95% CI 3.6% to 6.0%) in eight studies (n=10 199), which performed multiple ECG recordings was comparable to that with 24 hours Holter (4.6%, 95% CI 3.5% to 5.7%). Intermittent recordings for 19 min total produced similar AF detection to 24 hours Holter monitoring.
Conclusion: Portable ECG devices may offer an efficient screening option for AF compared with 24 hours Holter monitoring.
Prospero registration number: CRD42017061021.
© Author(s) (or their employer(s)) 2018. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SR receives equipment and software support from Semacare, a manufacturer of handheld ECG devices. Receives research scholarships from the Heart Foundation and Avant. THM receives equipment and software support from Semacare.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
