A Trihelix Family Transcription Factor Is Associated with Key Genes in Mixed-Linkage Glucan Accumulation
- PMID: 30224432
- PMCID: PMC6236600
- DOI: 10.1104/pp.18.00978
A Trihelix Family Transcription Factor Is Associated with Key Genes in Mixed-Linkage Glucan Accumulation
Abstract
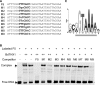
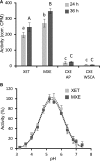
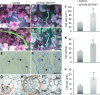
Mixed-linkage glucan (MLG) is a polysaccharide that is highly abundant in grass endosperm cell walls and present at lower amounts in other tissues. Cellulose synthase-like F (CSLF) and cellulose synthase-like H genes synthesize MLG, but it is unknown if other genes participate in the production and restructuring of MLG. Using Brachypodium distachyon transcriptional profiling data, we identified a B distachyon trihelix family transcription factor (BdTHX1) that is highly coexpressed with the B distachyon CSLF6 gene (BdCSLF6), which suggests that BdTHX1 is involved in the regulation of MLG biosynthesis. To determine the genes regulated by this transcription factor, we conducted chromatin immunoprecipitation sequencing (ChIP-seq) experiments using immature B distachyon seeds and an anti-BdTHX1 polyclonal antibody. The ChIP-seq experiment identified the second intron of BdCSLF6 as one of the most enriched sequences. The binding of BdTHX1 to the BdCSLF6 intron sequence was confirmed using electrophoretic mobility shift assays (EMSA). ChIP-seq also showed that a gene encoding a grass-specific glycoside hydrolase family 16 endotransglucosylase/hydrolase (BdXTH8) is bound by BdTHX1, and the binding was confirmed by EMSA. Radiochemical transglucanase assays showed that BdXTH8 exhibits predominantly MLG:xyloglucan endotransglucosylase activity, a hetero-transglycosylation reaction, and can thus produce MLG-xyloglucan covalent bonds; it also has a lower xyloglucan:xyloglucan endotransglucosylase activity. B distachyon shoots regenerated from transformed calli overexpressing BdTHX1 showed an abnormal arrangement of vascular tissue and seedling-lethal phenotypes. These results indicate that the transcription factor BdTHX1 likely plays an important role in MLG biosynthesis and restructuring by regulating the expression of BdCSLF6 and BdXTH8.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures




References
-
- Bailey TL, Elkan C (1994) Fitting a Mixture Model by Expectation Maximization to Discover Motifs in Biopolymers. Proc Second Int Conf Intell Syst Mol Biol. 28–36 - PubMed
-
- Bianchi M, Crinelli R, Giacomini E, Carloni E, Magnani M (2009) A potent enhancer element in the 5′-UTR intron is crucial for transcriptional regulation of the human ubiquitin C gene. Gene 448: 88–101 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

