Fibroblasts lacking nuclear lamins do not have nuclear blebs or protrusions but nevertheless have frequent nuclear membrane ruptures
- PMID: 30224463
- PMCID: PMC6176609
- DOI: 10.1073/pnas.1812622115
Fibroblasts lacking nuclear lamins do not have nuclear blebs or protrusions but nevertheless have frequent nuclear membrane ruptures
Abstract
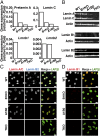
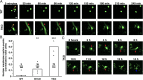
The nuclear lamina, an intermediate filament meshwork lining the inner nuclear membrane, is formed by the nuclear lamins (lamins A, C, B1, and B2). Defects or deficiencies in individual nuclear lamin proteins have been reported to elicit nuclear blebs (protrusions or outpouchings of the nuclear envelope) and increase susceptibility for nuclear membrane ruptures. It is unclear, however, how a complete absence of nuclear lamins would affect nuclear envelope morphology and nuclear membrane integrity (i.e., whether nuclear membrane blebs or protrusions would occur and, if not, whether cells would be susceptible to nuclear membrane ruptures). To address these issues, we generated mouse embryonic fibroblasts (MEFs) lacking all nuclear lamins. The nuclear lamin-deficient MEFs had irregular nuclear shapes but no nuclear blebs or protrusions. Despite a virtual absence of nuclear blebs, MEFs lacking nuclear lamins had frequent, prolonged, and occasionally nonhealing nuclear membrane ruptures. By transmission electron microscopy, the inner nuclear membrane in nuclear lamin-deficient MEFs have a "wavy" appearance, and there were discrete discontinuities in the inner and outer nuclear membranes. Nuclear membrane ruptures were accompanied by a large increase in DNA damage, as judged by γ-H2AX foci. Mechanical stress increased both nuclear membrane ruptures and DNA damage, whereas minimizing transmission of cytoskeletal forces to the nucleus had the opposite effects.
Keywords: nuclear envelope; nuclear lamina; nuclear membrane rupture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Burke B, Stewart CL. The nuclear lamins: Flexibility in function. Nat Rev Mol Cell Biol. 2013;14:13–24. - PubMed
-
- Moir RD, et al. Review: The dynamics of the nuclear lamins during the cell cycle—relationship between structure and function. J Struct Biol. 2000;129:324–334. - PubMed
-
- Ellis DJ, Jenkins H, Whitfield WG, Hutchison CJ. GST-lamin fusion proteins act as dominant negative mutants in Xenopus egg extract and reveal the function of the lamina in DNA replication. J Cell Sci. 1997;110:2507–2518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

