Development of a novel Hsp90 inhibitor NCT-50 as a potential anticancer agent for the treatment of non-small cell lung cancer
- PMID: 30224681
- PMCID: PMC6141536
- DOI: 10.1038/s41598-018-32196-6
Development of a novel Hsp90 inhibitor NCT-50 as a potential anticancer agent for the treatment of non-small cell lung cancer
Abstract
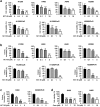
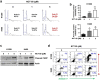
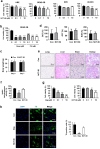
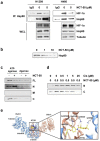
Despite the development of advanced therapeutic regimens such as molecular targeted therapy and immunotherapy, the 5-year survival of patients with lung cancer is still less than 20%, suggesting the need to develop additional treatment strategies. The molecular chaperone heat shock protein 90 (Hsp90) plays important roles in the maturation of oncogenic proteins and thus has been considered as an anticancer therapeutic target. Here we show the efficacy and biological mechanism of a Hsp90 inhibitor NCT-50, a novobiocin-deguelin analog hybridizing the pharmacophores of these known Hsp90 inhibitors. NCT-50 exhibited significant inhibitory effects on the viability and colony formation of non-small cell lung cancer (NSCLC) cells and those carrying resistance to chemotherapy. In contrast, NCT-50 showed minimal effects on the viability of normal cells. NCT-50 induced apoptosis in NSCLC cells, inhibited the expression and activity of several Hsp90 clients including hypoxia-inducible factor (HIF)-1α, and suppressed pro-angiogenic effects of NSCLC cells. Further biochemical and in silico studies revealed that NCT-50 downregulated Hsp90 function by interacting with the C-terminal ATP-binding pocket of Hsp90, leading to decrease in the interaction with Hsp90 client proteins. These results suggest the potential of NCT-50 as an anticancer Hsp90 inhibitor.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

