T cell receptor cross-reactivity expanded by dramatic peptide-MHC adaptability
- PMID: 30224695
- PMCID: PMC6371774
- DOI: 10.1038/s41589-018-0130-4
T cell receptor cross-reactivity expanded by dramatic peptide-MHC adaptability
Abstract
T cell receptor cross-reactivity allows a fixed T cell repertoire to respond to a much larger universe of potential antigens. Recent work has emphasized the importance of peptide structural and chemical homology, as opposed to sequence similarity, in T cell receptor cross-reactivity. Surprisingly, though, T cell receptors can also cross-react between ligands with little physiochemical commonalities. Studying the clinically relevant receptor DMF5, we demonstrate that cross-recognition of such divergent antigens can occur through mechanisms that involve heretofore unanticipated rearrangements in the peptide and presenting MHC protein, including binding-induced peptide register shifts and extensions from MHC peptide binding grooves. Moreover, cross-reactivity can proceed even when such dramatic rearrangements do not translate into structural or chemical molecular mimicry. Beyond demonstrating new principles of T cell receptor cross-reactivity, our results have implications for efforts to predict and control T cell specificity and cross-reactivity and highlight challenges associated with predicting T cell reactivities.
Conflict of interest statement
Competing financial interests
T.P.R. is employed by a new startup company that uses structural information to explore and modulate TCR specificity. B.M.B. is on the board of this company.
Figures



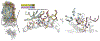
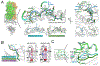

Comment in
-
Flipping out the peptide.Nat Chem Biol. 2018 Oct;14(10):905-906. doi: 10.1038/s41589-018-0133-1. Nat Chem Biol. 2018. PMID: 30224693 No abstract available.
References
-
- Mason D A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunology Today 19, 395–404 (1998). - PubMed
Online Methods References
-
- Das R & Baker D Macromolecular modeling with rosetta. Annu Rev Biochem 77, 363–382 (2008). - PubMed
-
- Davis-Harrison RL, Armstrong KM & Baker BM Two Different T Cell Receptors use Different Thermodynamic Strategies to Recognize the Same Peptide/MHC Ligand. Journal of Molecular Biology 346, 533–550 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

