Kidney-resident macrophages promote a proangiogenic environment in the normal and chronically ischemic mouse kidney
- PMID: 30224726
- PMCID: PMC6141464
- DOI: 10.1038/s41598-018-31887-4
Kidney-resident macrophages promote a proangiogenic environment in the normal and chronically ischemic mouse kidney
Abstract
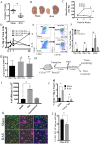
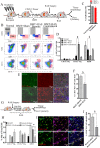
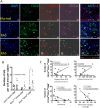
Renal artery stenosis (RAS) caused by narrowing of arteries is characterized by microvascular damage. Macrophages are implicated in repair and injury, but the specific populations responsible for these divergent roles have not been identified. Here, we characterized murine kidney F4/80+CD64+ macrophages in three transcriptionally unique populations. Using fate-mapping and parabiosis studies, we demonstrate that CD11b/cint are long-lived kidney-resident (KRM) while CD11chiMϕ, CD11cloMϕ are monocyte-derived macrophages. In a murine model of RAS, KRM self-renewed, while CD11chiMϕ and CD11cloMϕ increased significantly, which was associated with loss of peritubular capillaries. Replacing the native KRM with monocyte-derived KRM using liposomal clodronate and bone marrow transplantation followed by RAS, amplified loss of peritubular capillaries. To further elucidate the nature of interactions between KRM and peritubular endothelial cells, we performed RNA-sequencing on flow-sorted macrophages from Sham and RAS kidneys. KRM showed a prominent activation pattern in RAS with significant enrichment in reparative pathways, like angiogenesis and wound healing. In culture, KRM increased proliferation of renal peritubular endothelial cells implying direct pro-angiogenic properties. Human homologs of KRM identified as CD11bintCD11cintCD68+ increased in post-stenotic kidney biopsies from RAS patients compared to healthy human kidneys, and inversely correlated to kidney function. Thus, KRM may play protective roles in stenotic kidney injury through expansion and upregulation of pro-angiogenic pathways.
Conflict of interest statement
This work was partly funded by a research grant from Biogen. IL is a current Biogen employee, and JSD a former employee of Biogen (currently at Vertex Pharmaceuticals). Other author(s) have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016087/CA/NCI NIH HHS/United States
- HL123160/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)/International
- R01 DK102325/DK/NIDDK NIH HHS/United States
- U01 DK104273/DK/NIDDK NIH HHS/United States
- S10 OD021747/OD/NIH HHS/United States
- DK104273/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)/International
- R01 DK100081/DK/NIDDK NIH HHS/United States
- DK102325/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)/International
- R01 HL123160/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

