Differential regulation of chloride homeostasis and GABAergic transmission in the thalamus
- PMID: 30224811
- PMCID: PMC6141474
- DOI: 10.1038/s41598-018-31762-2
Differential regulation of chloride homeostasis and GABAergic transmission in the thalamus
Abstract
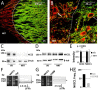
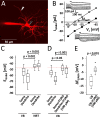
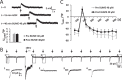
The thalamus is important for sensory integration with the ventrobasal thalamus (VB) as relay controlled by GABAergic projections from the nucleus reticularis thalami (NRT). Depending on the [Cl-]i primarily set by cation-chloride-cotransporters, GABA is inhibitory or excitatory. There is evidence that VB and NRT differ in terms of GABA action, with classical hyperpolarization in VB due to the expression of the Cl- extruder KCC2 and depolarizing/excitatory GABA action in the NRT, where KCC2 expression is low and Cl- accumulation by the Cl- inward transporter NKCC1 has been postulated. However, data on NKCC1 expression and functional analysis of both transporters are missing. We show that KCC2-mediated Cl- extrusion set the [Cl-]i in VB, while NKCC1 did not contribute substantially to Cl- accumulation and depolarizing GABA action in the NRT. The finding that NKCC1 did not play a major role in NRT neurons is of high relevance for ongoing studies on the therapeutic use of NKCC1 inhibitors trying to compensate for a disease-induced up-regulation of NKCC1 that has been described for various brain regions and disease states like epilepsy and chronic pain. These data suggest that NKCC1 inhibitors might have no major effect on healthy NRT neurons due to limited NKCC1 function.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Layer-specific expression of Cl- transporters and differential [Cl-]i in newborn rat cortex.Neuroreport. 2002 Dec 20;13(18):2433-7. doi: 10.1097/00001756-200212200-00012. Neuroreport. 2002. PMID: 12499844
-
Anomalous levels of Cl- transporters cause a decrease of GABAergic inhibition in human peritumoral epileptic cortex.Epilepsia. 2011 Sep;52(9):1635-44. doi: 10.1111/j.1528-1167.2011.03111.x. Epub 2011 Jun 2. Epilepsia. 2011. PMID: 21635237
-
Evidence for Two Subpopulations of Cerebrospinal Fluid-Contacting Neurons with Opposite GABAergic Signaling in Adult Mouse Spinal Cord.J Neurosci. 2024 May 29;44(22):e2289222024. doi: 10.1523/JNEUROSCI.2289-22.2024. J Neurosci. 2024. PMID: 38684364 Free PMC article.
-
Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia.Curr Top Med Chem. 2005;5(6):547-55. doi: 10.2174/1568026054367629. Curr Top Med Chem. 2005. PMID: 16022677 Free PMC article. Review.
-
NKCC1, an Elusive Molecular Target in Brain Development: Making Sense of the Existing Data.Cells. 2020 Dec 4;9(12):2607. doi: 10.3390/cells9122607. Cells. 2020. PMID: 33291778 Free PMC article. Review.
Cited by
-
The Therapeutic Potential of Neuronal K-Cl Co-Transporter KCC2 in Huntington's Disease and Its Comorbidities.Int J Mol Sci. 2020 Nov 30;21(23):9142. doi: 10.3390/ijms21239142. Int J Mol Sci. 2020. PMID: 33266310 Free PMC article. Review.
-
Neuronal K+-Cl- cotransporter KCC2 as a promising drug target for epilepsy treatment.Acta Pharmacol Sin. 2024 Jan;45(1):1-22. doi: 10.1038/s41401-023-01149-9. Epub 2023 Sep 13. Acta Pharmacol Sin. 2024. PMID: 37704745 Free PMC article. Review.
-
Intricacies of GABAA Receptor Function: The Critical Role of the β3 Subunit in Norm and Pathology.Int J Mol Sci. 2021 Feb 1;22(3):1457. doi: 10.3390/ijms22031457. Int J Mol Sci. 2021. PMID: 33535681 Free PMC article. Review.
-
Massive Activation of GABAA Receptors: Rundown, Ionic and Neurodegenerative Consequences.Biomolecules. 2025 Jul 13;15(7):1003. doi: 10.3390/biom15071003. Biomolecules. 2025. PMID: 40723875 Free PMC article. Review.
-
Role of the cation-chloride-cotransporters in the circadian system.Asian J Pharm Sci. 2021 Sep;16(5):589-597. doi: 10.1016/j.ajps.2020.10.003. Epub 2020 Dec 7. Asian J Pharm Sci. 2021. PMID: 34849164 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

