Effects of aging on timing of hibernation and reproduction
- PMID: 30224823
- PMCID: PMC6141465
- DOI: 10.1038/s41598-018-32311-7
Effects of aging on timing of hibernation and reproduction
Erratum in
-
Author Correction: Effects of aging on timing of hibernation and reproduction.Sci Rep. 2019 Oct 24;9(1):15553. doi: 10.1038/s41598-019-51736-2. Sci Rep. 2019. PMID: 31645665 Free PMC article.
Abstract
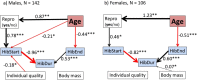
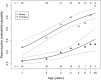
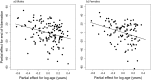
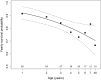
Small hibernators are long-lived for their size because seasonal dormancy greatly reduces predation risk. Thus, within a year, hibernators switch between states of contrasting mortality risk (active season versus hibernation), making them interesting species for testing the predictions of life-history theory. Accordingly, we hypothesized that, with advancing age and hence diminishing reproductive potential, hibernators should increasingly accept the higher predation risk associated with activity to increase the likelihood of current reproductive success. For edible dormice (Glis glis) we show that age strongly affects hibernation/activity patterns, and that this occurs via two pathways: (i) with increasing age, dormice are more likely to reproduce, which delays the onset of hibernation, and (ii) age directly advances emergence from hibernation in spring. We conclude that hibernation has to be viewed not merely as an energy saving strategy under harsh climatic conditions, but as an age-affected life-history trait that is flexibly used to maximize fitness.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Why hibernate? Predator avoidance in the edible dormouse.Mamm Res. 2023;68(1):1-11. doi: 10.1007/s13364-022-00652-4. Epub 2022 Oct 6. Mamm Res. 2023. PMID: 36624745 Free PMC article. Review.
-
It takes two to tango: Phagocyte and lymphocyte numbers in a small mammalian hibernator.Brain Behav Immun. 2016 Feb;52:71-80. doi: 10.1016/j.bbi.2015.09.018. Epub 2015 Oct 9. Brain Behav Immun. 2016. PMID: 26431693
-
Telomere dynamics in free-living edible dormice (Glis glis): the impact of hibernation and food supply.J Exp Biol. 2016 Aug 15;219(Pt 16):2469-74. doi: 10.1242/jeb.140871. J Exp Biol. 2016. PMID: 27535986 Free PMC article.
-
How to spend the summer? Free-living dormice (Glis glis) can hibernate for 11 months in non-reproductive years.J Comp Physiol B. 2015 Dec;185(8):931-9. doi: 10.1007/s00360-015-0929-1. Epub 2015 Aug 21. J Comp Physiol B. 2015. PMID: 26293446 Free PMC article.
-
Physiological, Behavioral, and Life-History Adaptations to Environmental Fluctuations in the Edible Dormouse.Front Physiol. 2020 May 5;11:423. doi: 10.3389/fphys.2020.00423. eCollection 2020. Front Physiol. 2020. PMID: 32431626 Free PMC article. Review.
Cited by
-
Evolutionary trade-offs in dormancy phenology.Elife. 2024 Apr 26;12:RP89644. doi: 10.7554/eLife.89644. Elife. 2024. PMID: 38669069 Free PMC article.
-
Flexible energy-saving strategies in female temperate-zone bats.J Comp Physiol B. 2022 Nov;192(6):805-814. doi: 10.1007/s00360-022-01452-7. Epub 2022 Aug 8. J Comp Physiol B. 2022. PMID: 35939092 Free PMC article.
-
Safe Periods and Safe Activities: Two Phenological Responses to Mortality.Ecol Evol. 2025 Feb 2;15(2):e70718. doi: 10.1002/ece3.70718. eCollection 2025 Feb. Ecol Evol. 2025. PMID: 39901893 Free PMC article. Review.
-
The Torpid State: Recent Advances in Metabolic Adaptations and Protective Mechanisms†.Front Physiol. 2021 Jan 20;11:623665. doi: 10.3389/fphys.2020.623665. eCollection 2020. Front Physiol. 2021. PMID: 33551846 Free PMC article. Review.
-
Why hibernate? Predator avoidance in the edible dormouse.Mamm Res. 2023;68(1):1-11. doi: 10.1007/s13364-022-00652-4. Epub 2022 Oct 6. Mamm Res. 2023. PMID: 36624745 Free PMC article. Review.
References
-
- Dobson, F. S. & Oli, M. K. Fast and slow life histories of mammals. Ecoscience14, 292–299, doi:10.2980/1195-6860(2007)14[292:FASLHO]2.0.CO;2 (2007).
-
- Sherman PW, Jarvis JUM. Extraordinary life spans of naked mole-rats (Heterocephalus glaber) J Zool. 2002;258:307–311. doi: 10.1017/S0952836902001437. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

