MT1-MMP as a PET Imaging Biomarker for Pancreas Cancer Management
- PMID: 30224904
- PMCID: PMC6129362
- DOI: 10.1155/2018/8382148
MT1-MMP as a PET Imaging Biomarker for Pancreas Cancer Management
Abstract
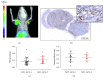
Pancreatic ductal adenocarcinoma (PDAC) continues to be one of the deadliest cancers for which optimal diagnostic tools are still greatly needed. Identification of PDAC-specific molecular markers would be extremely useful to improve disease diagnosis and follow-up. MT1-MMP has long been involved in pancreatic cancer, especially in tumour invasion and metastasis. In this study, we aim to ascertain the suitability of MT1-MMP as a biomarker for positron emission tomography (PET) imaging. Two probes were assessed and compared for this purpose, an MT1-MMP-specific binding peptide (MT1-AF7p) and a specific antibody (LEM2/15), labelled, respectively, with 68Ga and with 89Zr. PET imaging with both probes was conducted in patient-derived xenograft (PDX), subcutaneous and orthotopic, PDAC mouse models, and in a cancer cell line (CAPAN-2)-derived xenograft (CDX) model. Both radiolabelled tracers were successful in identifying, by means of PET imaging techniques, tumour tissues expressing MT1-MMP although they did so at different uptake levels. The 89Zr-DFO-LEM2/15 probe showed greater specific activity compared to the 68Ga-labelled peptide. The mean value of tumour uptake for the 89Zr-DFO-LEM2/15 probe (5.67 ± 1.11%ID/g, n=28) was 25-30 times higher than that of the 68Ga-DOTA-AF7p ones. Tumour/blood ratios (1.13 ± 0.51 and 1.44 ± 0.43 at 5 and 7 days of 89Zr-DFO-LEM2/15 after injection) were higher than those estimated for 68Ga-DOTA-AF7p probes (of approximately tumour/blood ratio = 0.5 at 90 min after injection). Our findings strongly point out that (i) the in vivo detection of MT1-MMP by PET imaging is a promising strategy for PDAC diagnosis and (ii) labelled LEM2/15 antibody is a better candidate than MT1-AF7p for PDAC detection.
Figures

 ) and 4°C (
) and 4°C ( ) and human plasma at 37°C (
) and human plasma at 37°C ( ) and 4°C (
) and 4°C ( ); data expressed as mean ± SD. (n=4). Representative HPLC chromatogram of 68Ga-DOTA-AF7p-1 before (c) or after (d) to Sep-Pak Light C18 cartridge purification. 68Ga-DOTA-AF7p-1 is detected at 8.7 min by UV absorbance at 280 nm (
); data expressed as mean ± SD. (n=4). Representative HPLC chromatogram of 68Ga-DOTA-AF7p-1 before (c) or after (d) to Sep-Pak Light C18 cartridge purification. 68Ga-DOTA-AF7p-1 is detected at 8.7 min by UV absorbance at 280 nm ( ) and radioactivity detector (
) and radioactivity detector ( ).
).
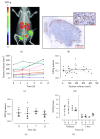
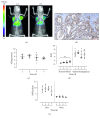
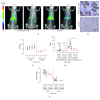
Similar articles
-
Target engagement of an anti-MT1-MMP antibody for triple-negative breast cancer PET imaging and beta therapy.Nucl Med Biol. 2024 Sep-Oct;136-137:108930. doi: 10.1016/j.nucmedbio.2024.108930. Epub 2024 May 23. Nucl Med Biol. 2024. PMID: 38833768
-
Targeting MT1-MMP as an ImmunoPET-Based Strategy for Imaging Gliomas.PLoS One. 2016 Jul 27;11(7):e0158634. doi: 10.1371/journal.pone.0158634. eCollection 2016. PLoS One. 2016. PMID: 27462980 Free PMC article.
-
Site-specifically labeled 89Zr-DFO-trastuzumab improves immuno-reactivity and tumor uptake for immuno-PET in a subcutaneous HER2-positive xenograft mouse model.Theranostics. 2019 Jun 9;9(15):4409-4420. doi: 10.7150/thno.32883. eCollection 2019. Theranostics. 2019. PMID: 31285769 Free PMC article.
-
Current knowledge on the sensitivity of the (68)Ga-somatostatin receptor positron emission tomography and the SUVmax reference range for management of pancreatic neuroendocrine tumours.Eur J Nucl Med Mol Imaging. 2016 Oct;43(11):2072-83. doi: 10.1007/s00259-016-3395-4. Epub 2016 May 13. Eur J Nucl Med Mol Imaging. 2016. PMID: 27174220 Free PMC article. Review.
-
Molecular Targeted Positron Emission Tomography Imaging and Radionuclide Therapy of Pancreatic Ductal Adenocarcinoma.Cancers (Basel). 2021 Dec 7;13(24):6164. doi: 10.3390/cancers13246164. Cancers (Basel). 2021. PMID: 34944781 Free PMC article. Review.
Cited by
-
ImmunoPET: Antibody-Based PET Imaging in Solid Tumors.Front Med (Lausanne). 2022 Jun 28;9:916693. doi: 10.3389/fmed.2022.916693. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35836956 Free PMC article. Review.
-
Pancreatic Ductal Adenocarcinoma: The Dawn of the Era of Nuclear Medicine?Int J Mol Sci. 2021 Jun 15;22(12):6413. doi: 10.3390/ijms22126413. Int J Mol Sci. 2021. PMID: 34203923 Free PMC article. Review.
-
ImmunoPET: Concept, Design, and Applications.Chem Rev. 2020 Apr 22;120(8):3787-3851. doi: 10.1021/acs.chemrev.9b00738. Epub 2020 Mar 23. Chem Rev. 2020. PMID: 32202104 Free PMC article. Review.
-
Development of anti-membrane type 1-matrix metalloproteinase nanobodies as immunoPET probes for triple negative breast cancer imaging.Front Med (Lausanne). 2022 Nov 24;9:1058455. doi: 10.3389/fmed.2022.1058455. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36507540 Free PMC article.
-
Overview and Future Perspectives on Tumor-Targeted Positron Emission Tomography and Fluorescence Imaging of Pancreatic Cancer in the Era of Neoadjuvant Therapy.Cancers (Basel). 2021 Dec 2;13(23):6088. doi: 10.3390/cancers13236088. Cancers (Basel). 2021. PMID: 34885196 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
