Safety and Efficacy of Ferula asafoetida in Functional Dyspepsia: A Randomized, Double-Blinded, Placebo-Controlled Study
- PMID: 30224930
- PMCID: PMC6129344
- DOI: 10.1155/2018/4813601
Safety and Efficacy of Ferula asafoetida in Functional Dyspepsia: A Randomized, Double-Blinded, Placebo-Controlled Study
Abstract
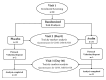
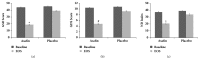
Despite the availability of various synthetic drugs for the treatment of functional dyspepsia (FD), the side effects and their cost have always created a great interest in the search for novel natural alternatives for the management of gut disorders. The present contribution reports the safety and efficacy of the kitchen spice asafoetida (Ferula asafoetida) in FD for the first time. In the double-blinded, placebo-controlled study, 43 subjects diagnosed to have moderate to severe discomforts of nonulcer FD were randomized to receive hard-shell capsules (250 mg × 2/day) of either placebo (n=22) or a food-grade formulation of asafoetida (Asafin) (n=21) for 30 days. When evaluated by a set of validated indexing tools (GSRS, GDSS, and NDI), almost 81% in the Asafin group showed significant (p < 0.01) improvement in the overall score and quality of life as compared to the placebo. At the end of the study, 66% of subjects in the Asafin group remained symptoms-free. Although the symptoms score improved significantly in both the groups (from -5.67 to -25.29 in Asafin group versus -1.55 to -6.0 in the placebo; p ≤ 0.001), the relative percentage of subjects in the Asafin group with more than 80% reduction in various symptoms were: bloating (58%), appetite (69%), postprandial fullness (74%) motion sickness (75%), and digestion (77%) as compared to less than 10% nonspecific improvement in the placebo group. All the subjects remained safe with no adverse events or variations in haematological and biochemical parameters. The study was registered at http://ctri.nic.in/ (CTRI/2018/ 01/011149).
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

