A feasibility study prior to an international multicentre paediatric study to assess pharmacokinetic/pharmacodynamic sampling and sample preparation procedures, logistics and bioanalysis
- PMID: 30225392
- PMCID: PMC6139604
- DOI: 10.1016/j.conctc.2018.08.008
A feasibility study prior to an international multicentre paediatric study to assess pharmacokinetic/pharmacodynamic sampling and sample preparation procedures, logistics and bioanalysis
Abstract
Background: Variability in pre-analytical procedures such as blood sampling, sample preparation and transport can substantially influence bioanalytical results and subsequently impair reliability of data gathered during clinical trials. Especially in vulnerable populations, all efforts should be made to facilitate high-quality data extraction excluding unnecessary or repeated intervention.
Methods: The EU-funded LENA project (Labeling of Enalapril from Neonates up to Adolescents) included a feasibility study in its preparatory procedures prior to first-in-child studies. Derived from a regular study visit, it encompassed all procedures, from sampling of two study-specific drugs and four sensitive humoral parameters to bioanalysis, to evaluate the quality of obtained samples and applicability of logistical and bioanalytical procedures. Drug administration to healthy adults was circumvented by pre-spiking the blood collection tubes with a drug solution. Five clinical sites were evaluated.
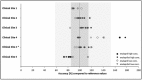
Results: Clinical teams' preparedness and applicability of required sampling procedures was investigated in 18 volunteers, on-site. 97% of collected pharmacokinetic (PK) samples and 93% of samples for humoral parameters were obtained eligibly. Results met expectations, though one team had to be re-trained and performed a re-run. Planned procedures for sampling, sample preparation, transport and analysis were found to be suitable for being applied within paediatric trials.
Conclusion: The concept of the presented feasibility study that simultaneously assesses PK/PD sampling, sample preparation, logistics and bioanalysis proved to be a promising tool for trial preparation. It revealed improperly installed processes and bottlenecks that required adjustments prior to start of recruitment. It facilitated high-quality conduct from the first moment of paediatric pivotal studies.
Keywords: ACE, Angiotensin-converting-enzyme; Clinical trial; Cmax, maximum serum concentration; ELISA, Enzyme-linked immunosorbent assay; EMA, European Medicines Agency; EU, European Union; FDA, U.S. Food and Drug Administration; Feasibility; GCP, Good Clinical Practice; LC-MS/MS, Liquid chromatography-tandem mass spectrometry; LENA, Labeling of Enalapril from Neonates up to Adolescents; PD, Pharmacodynamic(s); PK, Pharmacokinetic(s); Pharmacodynamic; Pharmacokinetic; Pilot; RAA system, Renin-angiotensin-aldosterone system; RIA, Radioimmunoassay; Training concept; pp, Percentage points.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Teahan O., Gamble S., Holmes E., Waxman J., Nicholson J.K., Bevan C., Keun H.C. Impact of analytical bias in metabonomic studies of human blood serum and plasma. Anal. Chem. 2006;78(13):4307–4318. - PubMed
-
- Rai A.J., Vitzthum F. Effects of preanalytical variables on peptide and protein measurements in human serum and plasma: implications for clinical proteomics. Expet Rev. Proteonomics. 2006;3(4):409–426. - PubMed
-
- McGlasson D.L., Kaczor D.A., Krasuski R.A., Campbell C.L., Kostur M.R., Adinaro J.T. Effects of pre-analytical variables on the anti-activated factor X chromogenic assay when monitoring unfractionated heparin and low molecular weight heparin anticoagulation. Blood Coagulation & Fibrinolysis: An International Journal in Haemostasis and Thrombosis. 2005;16(3):173–176. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

