Impaired histaminergic neurotransmission in children with narcolepsy type 1
- PMID: 30225986
- PMCID: PMC6488909
- DOI: 10.1111/cns.13057
Impaired histaminergic neurotransmission in children with narcolepsy type 1
Abstract
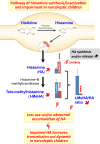
Objective: Narcolepsy is a sleep disorder characterized in humans by excessive daytime sleepiness and cataplexy. Greater than fifty percent of narcoleptic patients have an onset of symptoms prior to the age of 18. Current general agreement considers the loss of hypothalamic hypocretin (orexin) neurons as the direct cause of narcolepsy notably cataplexy. To assess whether brain histamine (HA) is also involved, we quantified the cerebrospinal fluid (CSF) levels of HA and tele-methylhistamine (t-MeHA), the direct metabolite of HA between children with orexin-deficient narcolepsy type 1 (NT1) and controls.
Methods: We included 24 children with NT1 (12.3 ± 3.6 years, 11 boys, 83% cataplexy, 100% HLA DQB1*06:02) and 21 control children (11.2 ± 4.2 years, 10 boys). CSF HA and t-MeHA were measured in all subjects using a highly sensitive liquid chromatographic-electrospray/tandem mass spectrometric assay. CSF hypocretin-1 values were determined in the narcoleptic patients.
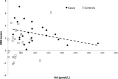
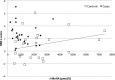
Results: Compared with the controls, NT1 children had higher CSF HA levels (771 vs 234 pmol/L, P < 0.001), lower t-MeHA levels (879 vs 1924 pmol/L, P < 0.001), and lower t-MeHA/HA ratios (1.1 vs 8.2, P < 0.001). NT1 patients had higher BMI z-scores (2.7 ± 1.6 vs 1.0 ± 2.3, P = 0.006) and were more often obese (58% vs 29%, P = 0.05) than the controls. Multivariable analyses including age, gender, and BMI z-score showed a significant decrease in CSF HA levels when the BMI z-score increased in patients (P = 0.007) but not in the controls. No association was found between CSF HA, t-MeHA, disease duration, age at disease onset, the presence of cataplexy, lumbar puncture timing, and CSF hypocretin levels.
Conclusions: Narcolepsy type 1 children had a higher CSF HA level together with a lower t-MeHA level leading to a significant decrease in the t-MeHA/HA ratios. These results suggest a decreased HA turnover and an impairment of histaminergic neurotransmission in narcoleptic children and support the use of a histaminergic therapy in the treatment against narcolepsy.
Keywords: histamine; hypocretin (orexin); narcolepsy; pediatrics; sleep.
© 2018 John Wiley & Sons Ltd.
Conflict of interest statement
Pr Yves Dauvilliers received funds for speaking and board engagements with UCB pharma, Jazz, Flamel, Theranexus, GSK, and Bioprojet. He was investigator in studies promoted by Bioprojet (Pitolisant). Pr Patricia Franco received funds for speaking and board engagements with UCB pharma and Procter & Gamble. She is investigator in clinical studies promoted by Bioprojet (Pitolisant). Pr Jian‐Sheng Lin received funds for speaking with Bioprojet. The other authors have nothing to report.
Figures

Similar articles
-
Normal cerebrospinal fluid histamine and tele-methylhistamine levels in hypersomnia conditions.Sleep. 2012 Oct 1;35(10):1359-66. doi: 10.5665/sleep.2114. Sleep. 2012. PMID: 23024434 Free PMC article.
-
Temporal Changes in the Cerebrospinal Fluid Level of Hypocretin-1 and Histamine in Narcolepsy.Sleep. 2017 Jan 1;40(1):zsw010. doi: 10.1093/sleep/zsw010. Sleep. 2017. PMID: 28364477 Free PMC article.
-
Decreased CSF histamine in narcolepsy with and without low CSF hypocretin-1 in comparison to healthy controls.Sleep. 2009 Feb;32(2):175-80. doi: 10.1093/sleep/32.2.175. Sleep. 2009. PMID: 19238804 Free PMC article.
-
Symptomatic narcolepsy, cataplexy and hypersomnia, and their implications in the hypothalamic hypocretin/orexin system.Sleep Med Rev. 2005 Aug;9(4):269-310. doi: 10.1016/j.smrv.2005.03.004. Sleep Med Rev. 2005. PMID: 16006155 Review.
-
Narcolepsies, update in 2023.Rev Neurol (Paris). 2023 Oct;179(7):727-740. doi: 10.1016/j.neurol.2023.08.001. Epub 2023 Aug 25. Rev Neurol (Paris). 2023. PMID: 37634997 Review.
Cited by
-
Body Weight and Metabolic Rate Changes in Narcolepsy: Current Knowledge and Future Directions.Metabolites. 2022 Nov 16;12(11):1120. doi: 10.3390/metabo12111120. Metabolites. 2022. PMID: 36422261 Free PMC article. Review.
-
Whole-brain mapping of histaminergic projections in mouse brain.Proc Natl Acad Sci U S A. 2023 Apr 4;120(14):e2216231120. doi: 10.1073/pnas.2216231120. Epub 2023 Mar 28. Proc Natl Acad Sci U S A. 2023. PMID: 36976764 Free PMC article.
-
Histamine in murine narcolepsy: What do genetic and immune models tell us?Brain Pathol. 2022 Mar;32(2):e13027. doi: 10.1111/bpa.13027. Epub 2021 Oct 21. Brain Pathol. 2022. PMID: 34672414 Free PMC article.
-
Orexin/Hypocretin and Histamine Cross-Talk on Hypothalamic Neuron Counts in Mice.Front Neurosci. 2021 May 20;15:660518. doi: 10.3389/fnins.2021.660518. eCollection 2021. Front Neurosci. 2021. PMID: 34093114 Free PMC article.
-
The Histaminergic System in Neuropsychiatric Disorders.Biomolecules. 2021 Sep 11;11(9):1345. doi: 10.3390/biom11091345. Biomolecules. 2021. PMID: 34572558 Free PMC article. Review.
References
-
- American Academy of Sleep Medicine . International Classification of Sleep Disorders – Third Edition (ICSD‐3). Darien, IL: American Academy of Sleep Medicine; 2014.
-
- Kornum BR, Knudsen S, Ollila HM, et al. Narcolepsy. Nat Rev Dis Primers. 2017;3:16100. - PubMed
-
- Valko PO, Gavrilov YV, Yamamoto M, et al. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann Neurol. 2013;74:794‐804. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

