Adipose Tissue Dysfunction Occurs Independently of Obesity in Adipocyte-Specific Oncostatin Receptor Knockout Mice
- PMID: 30226002
- PMCID: PMC6146404
- DOI: 10.1002/oby.22254
Adipose Tissue Dysfunction Occurs Independently of Obesity in Adipocyte-Specific Oncostatin Receptor Knockout Mice
Abstract
Objective: This study examined the phenotypic effects of adipocyte-specific oncostatin M receptor (OSMR) loss in chow-fed mice.
Methods: Chow-fed adipocyte-specific OSMR knockout (FKO) mice and littermate OSMRfl/fl controls were studied. Tissue weights, insulin sensitivity, adipokine production, and stromal cell immunophenotypes were assessed in epididymal fat (eWAT); serum adipokine production was also assessed. In vitro, adipocytes were treated with oncostatin M, and adipokine gene expression was assessed.
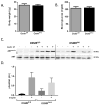
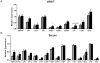
Results: Body weights, fasting blood glucose levels, and eWAT weights did not differ between genotypes. However, the eWAT of OSMRFKO mice was modestly less responsive to insulin stimulation than that of OSMRfl/fl mice. Notably, significant increases in adipokines, including C-reactive protein, lipocalin 2, intercellular adhesion molecule-1, and insulinlike growth factor binding protein 6, were observed in the eWAT of OSMRFKO mice. In addition, significant increases in fetuin A and intercellular adhesion molecule-1 were detected in OSMRFKO serum. Flow cytometry revealed a significant increase in leukocyte number and modest, but not statistically significant, increases in B cells and T cells in the eWAT of OSMRFKO mice.
Conclusions: The chow-fed OSMRFKO mice exhibited adipose tissue dysfunction and increased proinflammatory adipokine production. These results suggest that intact adipocyte oncostatin M-OSMR signaling is necessary for adipose tissue immune cell homeostasis.
© 2018 The Obesity Society.
Conflict of interest statement
Figures
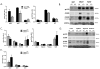
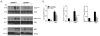
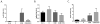
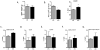
Similar articles
-
Oncostatin M Induces Lipolysis and Suppresses Insulin Response in 3T3-L1 Adipocytes.Int J Mol Sci. 2022 Apr 23;23(9):4689. doi: 10.3390/ijms23094689. Int J Mol Sci. 2022. PMID: 35563078 Free PMC article.
-
Loss of Oncostatin M Signaling in Adipocytes Induces Insulin Resistance and Adipose Tissue Inflammation in Vivo.J Biol Chem. 2016 Aug 12;291(33):17066-76. doi: 10.1074/jbc.M116.739110. Epub 2016 Jun 20. J Biol Chem. 2016. PMID: 27325693 Free PMC article.
-
Oncostatin M Mediates Adipocyte Expression and Secretion of Stromal-Derived Factor 1.Biology (Basel). 2019 Mar 23;8(1):19. doi: 10.3390/biology8010019. Biology (Basel). 2019. PMID: 30909581 Free PMC article.
-
Adipocyte Oncostatin Receptor Regulates Adipose Tissue Homeostasis and Inflammation.Front Immunol. 2021 Mar 29;11:612013. doi: 10.3389/fimmu.2020.612013. eCollection 2020. Front Immunol. 2021. PMID: 33854494 Free PMC article. Review.
-
Adipocyte-specific Hypoxia-inducible gene 2 promotes fat deposition and diet-induced insulin resistance.Mol Metab. 2016 Sep 28;5(12):1149-1161. doi: 10.1016/j.molmet.2016.09.009. eCollection 2016 Dec. Mol Metab. 2016. PMID: 27900258 Free PMC article.
Cited by
-
Adipose-tissue Treg cells restrain differentiation of stromal adipocyte precursors to promote insulin sensitivity and metabolic homeostasis.Immunity. 2024 Jun 11;57(6):1345-1359.e5. doi: 10.1016/j.immuni.2024.04.002. Epub 2024 Apr 30. Immunity. 2024. PMID: 38692280 Free PMC article.
-
Oncostatin M Induces Lipolysis and Suppresses Insulin Response in 3T3-L1 Adipocytes.Int J Mol Sci. 2022 Apr 23;23(9):4689. doi: 10.3390/ijms23094689. Int J Mol Sci. 2022. PMID: 35563078 Free PMC article.
-
Implications of Adipose Tissue Content for Changes in Serum Levels of Exercise-Induced Adipokines: A Quasi-Experimental Study.Int J Environ Res Public Health. 2022 Jul 19;19(14):8782. doi: 10.3390/ijerph19148782. Int J Environ Res Public Health. 2022. PMID: 35886639 Free PMC article.
-
Serum proteomic correlates of mental health symptoms in a representative UK population sample.Brain Behav Immun Health. 2025 Jan 15;44:100947. doi: 10.1016/j.bbih.2025.100947. eCollection 2025 Mar. Brain Behav Immun Health. 2025. PMID: 39911945 Free PMC article.
-
Oncostatin M promotes lipolysis in white adipocytes.Adipocyte. 2022 Dec;11(1):315-324. doi: 10.1080/21623945.2022.2075129. Adipocyte. 2022. PMID: 35531859 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

