Tuberculosis Treatment Monitoring by Video Directly Observed Therapy in 5 Health Districts, California, USA
- PMID: 30226154
- PMCID: PMC6154139
- DOI: 10.3201/eid2410.180459
Tuberculosis Treatment Monitoring by Video Directly Observed Therapy in 5 Health Districts, California, USA
Abstract
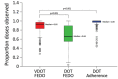
We assessed video directly observed therapy (VDOT) for monitoring tuberculosis treatment in 5 health districts in California, USA, to compare adherence between 174 patients using VDOT and 159 patients using in-person directly observed therapy (DOT). Multivariable linear regression analyses identified participant-reported sociodemographics, risk behaviors, and treatment experience associated with adherence. Median participant age was 44 (range 18-87) years; 61% of participants were male. Median fraction of expected doses observed (FEDO) among VDOT participants was higher (93.0% [interquartile range (IQR) 83.4%-97.1%]) than among patients receiving DOT (66.4% [IQR 55.1%-89.3%]). Most participants (96%) would recommend VDOT to others; 90% preferred VDOT over DOT. Lower FEDO was independently associated with US or Mexico birth, shorter VDOT duration, finding VDOT difficult, frequently taking medications while away from home, and having video-recording problems (p<0.05). VDOT cost 32% (range 6%-46%) less than DOT. VDOT was feasible, acceptable, and achieved high adherence at lower cost than DOT.
Keywords: California; United States; VDOT; antimicrobial resistance; bacteria; mHealth; medication adherence monitoring; patient-centered care; smartphone; tuberculosis and other mycobacteria; video directly observed therapy; video technology.
Figures

References
-
- Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis. 2016;63:e147–95. 10.1093/cid/ciw376 - DOI - PMC - PubMed
-
- Falzon D, Gandhi N, Migliori GB, Sotgiu G, Cox HS, Holtz TH, et al.; Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J. 2013;42:156–68. 10.1183/09031936.00134712 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

