Intermediates and Mechanism in Iron-Catalyzed Cross-Coupling
- PMID: 30226380
- PMCID: PMC6217991
- DOI: 10.1021/jacs.8b06893
Intermediates and Mechanism in Iron-Catalyzed Cross-Coupling
Abstract
Iron-catalyzed cross-coupling reactions have attracted significant research interest, as they offer numerous favorable features compared with cross-coupling reactions with precious metal catalysis. While this research has contributed to an empirical understanding of iron-catalyzed cross-coupling, the underlying fundamental mechanisms of reaction and structures of catalytically active species have remained poorly defined. The lack of such detail can be attributed to the difficulties associated with studying such iron-catalyzed reactions, where unstable paramagnetic intermediates abound. Recently, the combined application of physical-inorganic spectroscopic methods, concomitant organic product analysis, and air- and temperature-sensitive inorganic synthesis has yielded the most detailed insight currently available on reactivity and mechanism in iron-catalyzed cross-coupling. This Perspective highlights this approach and the limitations of the contributing techniques as well as some of the key features of the catalytic reactions studied and lessons learned.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


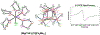

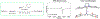




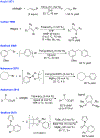

References
-
- Kharasch MS; Fields EK J. Am. Chem. Soc 1941, 63, 2316–2320.
-
- Tamura M; Kochi JK J. Am. Chem. Soc 1971, 93, 1487–1489.
-
- Neumann SM; Kochi JK J. Org. Chem 1975, 40, 599–606.
-
- Smith RS; Kochi JK J. Org. Chem 1976, 41, 502–509.
-
- Cahiez G; Avedissian H Synthesis 1998, 1998, 1199–1205.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

