Upregulation of macrophage migration inhibitory factor promotes tumor metastasis and correlates with poor prognosis of pancreatic ductal adenocarcinoma
- PMID: 30226561
- PMCID: PMC6151891
- DOI: 10.3892/or.2018.6703
Upregulation of macrophage migration inhibitory factor promotes tumor metastasis and correlates with poor prognosis of pancreatic ductal adenocarcinoma
Abstract
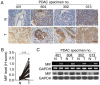
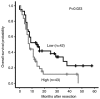
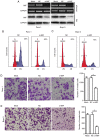
Macrophage migration inhibitory factor (MIF) is a pro‑inflammatory cytokine that serves important roles in cancer. MIF overexpression is frequently observed in numerous human cancer types, including pancreatic carcinoma. However, the prognostic value and function of MIF in pancreatic ductal adenocarcinoma (PDAC) have not been fully elucidated. In the present study, upregulation of MIF expression in PDAC tissue compared with adjacent normal tissue was observed. Furthermore, MIF overexpression was identified to be significantly associated with poor survival rates in patients with PDAC. Multivariate Cox regression analysis confirmed that MIF was an independent risk factor for poor survival. Functional analyses demonstrated that MIF knockdown significantly inhibited the proliferation and invasion of pancreatic cancer cells in vitro compared with control cells. IN addition, mechanistic investigations revealed that silencing MIF leads to inhibition of AKT serine/threonine kinase and extracellular‑signal‑regulated kinase activation, and suppression of cyclin D1 and matrix metalloproteinase‑2 expression, which may suppress tumor proliferation and invasion. These results highlight the importance of MIF overexpression in PDAC aggressiveness, and indicate that MIF may be a potential therapeutic target for pancreatic cancer.
Figures

References
-
- Liu SL, Friess H, Kleeff J, Ji ZL, Buchler MW. Surgical approaches for resection of pancreatic cancer: An overview. Hepatobiliary Pancreat Dis Int. 2002;1:118–125. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

