Inhibition of atypical protein kinase C‑ι effectively reduces the malignancy of prostate cancer cells by downregulating the NF-κB signaling cascade
- PMID: 30226591
- PMCID: PMC6192717
- DOI: 10.3892/ijo.2018.4542
Inhibition of atypical protein kinase C‑ι effectively reduces the malignancy of prostate cancer cells by downregulating the NF-κB signaling cascade
Abstract
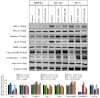
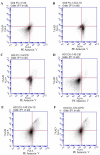
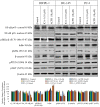
Prostate cancer (PC) is the most common type of cancer among men. Aggressive and metastatic PC results in life-threatening tumors, and represents one of the leading causes of mortality in men. Previous studies of atypical protein kinase C isoforms (aPKCs) have highlighted its role in the survival of cultured prostate cells via the nuclear factor (NF)-κB pathway. The present study showed that PKC‑ι was overexpressed in PC samples collected from cancer patients but not in non-invasive prostate tissues, indicating PKC‑ι as a possible prognostic biomarker for the progression of prostate carcinogenesis. Immunohistochemical staining further confirmed the association between PKC‑ι and the prostate malignancy. The DU‑145 and PC‑3 PC cell lines, and the non-neoplastic RWPE‑1 prostatic epithelial cell line were cultured and treated with aPKC inhibitors 2‑acetyl‑1,3-cyclopentanedione (ACPD) and 5-amino‑1-(1R,2S,3S,4R)-2,3-dihydroxy-4-methylcyclopentyl)‑1H-imidazole-4-carboxamide (ICA‑1). Western blot data demonstrated that ICA‑1 was an effective and specific inhibitor of PKC‑ι and that ACPD inhibited PKC‑ι and PKC‑ζ. Furthermore, the two inhibitors significantly decreased malignant cell proliferation and induced apoptosis. The inhibitors showed no significant cytotoxicity towards the RWPE‑1 cells, but exhibited cytostatic effects on the DU‑145 and PC‑3 cells prior to inducing apoptosis. The inhibition of aPKCs significantly reduced the translocation of NF-κB to the nucleus. Furthermore, this inhibition promoted apoptosis, reduced signaling for cell survival, and reduced the proliferation of PC cells, whereas the normal prostate epithelial cells were relatively unaffected. Overall, the results suggested that PKC‑ι and PKC‑ζ are essential for the progression of PC, and that ACPD and ICA‑1 can be effectively used as potential inhibitors in targeted therapy.
Figures




References
-
- American Cancer Society Key statistics for prostate cancer. Prostate Cancer Facts. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
-
- Kharaziha P, Chioureas D, Rutishauser D, Baltatzis G, Lennartsson L, Fonseca P, Azimi A, Hultenby K, Zubarev R, Ullén A, et al. Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget. 2015;6:21740–21754. doi: 10.18632/oncotarget.3226. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

