Blast-induced "PTSD": Evidence from an animal model
- PMID: 30227150
- PMCID: PMC12199260
- DOI: 10.1016/j.neuropharm.2018.09.023
Blast-induced "PTSD": Evidence from an animal model
Abstract
A striking observation among veterans returning from the recent conflicts in Iraq and Afghanistan has been the co-occurrence of blast-related mild traumatic brain injury (mTBI) and post-traumatic stress disorder (PTSD). PTSD and mTBI might coexist due to additive effects of independent psychological and physical traumas experienced in a war zone. Alternatively blast injury might induce PTSD-related traits or damage brain structures that mediate responses to psychological stressors, increasing the likelihood that PTSD will develop following a subsequent psychological stressor. Rats exposed to repetitive low-level blasts consisting of three 74.5 kPa exposures delivered once daily for three consecutive days develop a variety of anxiety and PTSD-related behavioral traits that are present for at least 9 months after blast exposure. A single predator scent challenge delivered 8 months after the last blast exposure induces additional anxiety-related changes that are still present 45 days later. Because the blast injuries occur under general anesthesia, it appears that blast exposure in the absence of a psychological stressor can induce chronic PTSD-related traits. The reaction to a predator scent challenge delivered many months after blast exposure suggests that blast exposure in addition sensitizes the brain to react abnormally to subsequent psychological stressors. The development of PTSD-related behavioral traits in the absence of a psychological stressor suggests the existence of blast-induced "PTSD". Findings that PTSD-related behavioral traits can be reversed by BCI-838, a group II metabotropic glutamate receptor antagonist offers insight into pathogenesis and possible treatment options for blast-related brain injury. This article is part of the Special Issue entitled "Novel Treatments for Traumatic Brain Injury".
Keywords: Animal models; Anxiety; BCI-838; Blast; Metabotropic glutamate receptor; Post-traumatic stress disorder; Postconcussion syndrome; Rat; Traumatic brain injury.
Published by Elsevier Ltd.
Conflict of interest statement
Conflicts of interest
None.
Figures


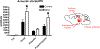



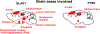
References
-
- Adamec R, Head D, Blundell J, Burton P, Berton O, 2006. Lasting anxiogenic effects of feline predator stress in mice: sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiol. Behav. 88, 12–29. - PubMed
-
- Ahlers ST, Vasserman-Stokes E, Shaughness MC, Hall AA, Shear DA, Chavko M, McCarron RM, Stone JR, 2012. Assessment of the effects of acute and repeated exposure to blast overpressure in rodents: toward a greater understanding of blast and the potential ramifications for injury in humans exposed to blast. Front. Neurol. 3, 32. - PMC - PubMed
-
- Bandelow B, Baldwin D, Abelli M, Altamura C, Dell’Osso B, Domschke K, Fineberg NA, Grunblatt E, Jarema M, Maron E, Nutt D, Pini S, Vaghi MM, Wichniak A, Zai G, Riederer P, 2016. Biological markers for anxiety disorders, OCD and PTSD - a consensus statement. Part I: neuroimaging and genetics. World J. Biol. Psychiatr. 17, 321–365. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

