Factors Secreted by Cancer-Associated Fibroblasts that Sustain Cancer Stem Properties in Head and Neck Squamous Carcinoma Cells as Potential Therapeutic Targets
- PMID: 30227608
- PMCID: PMC6162704
- DOI: 10.3390/cancers10090334
Factors Secreted by Cancer-Associated Fibroblasts that Sustain Cancer Stem Properties in Head and Neck Squamous Carcinoma Cells as Potential Therapeutic Targets
Abstract
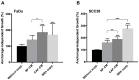
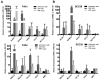
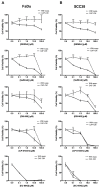
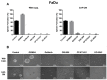
This study investigates for the first time the crosstalk between stromal fibroblasts and cancer stem cell (CSC) biology in head and neck squamous cell carcinomas (HNSCC), with the ultimate goal of identifying effective therapeutic targets. The effects of conditioned media from cancer-associated fibroblasts (CAFs) and normal fibroblasts (NFs) on the CSC phenotype were assessed by combining functional and expression analyses in HNSCC-derived cell lines. Further characterization of CAFs and NFs secretomes by mass spectrometry was followed by pharmacologic target inhibition. We demonstrate that factors secreted by CAFs but not NFs, in the absence of serum/supplements, robustly increased anchorage-independent growth, tumorsphere formation, and CSC-marker expression. Modulators of epidermal growth factor receptor (EGFR), insulin-like growth factor receptor (IGFR), and platelet-derived growth factor receptor (PDGFR) activity were identified as paracrine cytokines/factors differentially secreted between CAFs and NFs, in a mass spectrometry analysis. Furthermore, pharmacologic inhibition of EGFR, IGFR, and PDGFR significantly reduced CAF-induced tumorsphere formation and anchorage-independent growth suggesting a role of these receptor tyrosine kinases in sustaining the CSC phenotype. These findings provide novel insights into tumor stroma⁻CSC communication, and potential therapeutic targets to effectively block the CAF-enhanced CSC niche signaling circuit.
Keywords: cancer stem cells; cancer-associated fibroblasts; head and neck squamous cell carcinoma; secretome; therapeutic target; tumor microenvironment.
Conflict of interest statement
F.M. reports ownership of stock in EntreChem SL. All other authors declare they have no competing interests.
Figures


References
-
- Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R.P., Chaudhry S.I., Harrington K., Williamson P., Moeendarbary E., Charras G., et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. - DOI - PMC - PubMed
-
- Koczorowska M.M., Tholen S., Bucher F., Lutz L., Kizhakkedathu J.N., De Wever O., Wellner U.F., Biniossek M.L., Stahl A., Lassmann S., et al. Fibroblast activation protein-alpha, a stromal cell surface protease, shapes key features of cancer associated fibroblasts through proteome and degradome alterations. Mol. Oncol. 2016;10:40–58. doi: 10.1016/j.molonc.2015.08.001. - DOI - PMC - PubMed
-
- Bello I.O., Vered M., Dayan D., Dobriyan A., Yahalom R., Alanen K., Nieminen P., Kantola S., Laara E., Salo T. Cancer-associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma-associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol. 2011;47:33–38. doi: 10.1016/j.oraloncology.2010.10.013. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

