Sex-Mediated Differences in LPS Induced Alterations of TNFα, IL-10 Expression, and Prostaglandin Synthesis in Primary Astrocytes
- PMID: 30227622
- PMCID: PMC6164227
- DOI: 10.3390/ijms19092793
Sex-Mediated Differences in LPS Induced Alterations of TNFα, IL-10 Expression, and Prostaglandin Synthesis in Primary Astrocytes
Abstract
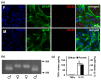
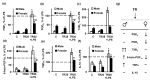
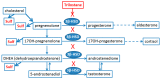
Although many neurological and psychiatric disorders reveal clear sex-dependent variations, the molecular mechanism of this process is not clear enough. Astrocytes are involved in the response of neural tissue to injury and inflammation, produce steroid hormones, and sense steroid presence. To explore the hypothesis that astrocytes may participate in sex-mediated differences of inflammatory responses, we have examined whether male and female primary rat astrocytes show different responses to lipopolysaccharide (LPS) as a toll-like receptor 4 (TLR4) agonist. Levels of mRNA and proteins of tumor necrosis factor alpha (TNFα), interleukin-10 (IL-10), and cyclooxygenase (COX)-2 were assessed using qPCR, immunoblotting, and ELISA. UPLC-MS/MS was used to detect prostaglandins (PGs). LPS stimulation resulted in different levels of cytokine production; more TNFα and less IL-10 were produced in female cells compared with male astrocytes. Although the levels of the COX-2 expression were not altered, LPS significantly induced the synthesis of PGs with notable sex-related differences. PGE₂ and PGD₂ were less and 6-keto-PGF1α was more upregulated in female astrocytes, and TXB₂ had similar levels in cells obtained from males and females. Trilostane, an inhibitor of 3β-Hydroxysteroid dehydrogenase (3β-HSD), inhibited the LPS-induced TNFα production and the release of PGE₂, PGD₂, and 6-keto-PGF1α in female astrocytes. Thus, male and female astrocytes differentially respond to inflammatory challenges on the level of production of cytokines and steroid hormones. Sex-mediated differences in pro- and anti-inflammatory responses should be taken into consideration for the effective treatment of disorders with neuroinflammation.
Keywords: 3β-HSD; COX-2; IL-10; LPS; TLR4; TNFα; astrocytes; neuroinflammation; sex difference; trilostane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

