Synthesis and Antisense Properties of 2'β-F-Arabinouridine Modified Oligonucleotides with 4'- C-OMe Substituent
- PMID: 30227644
- PMCID: PMC6225415
- DOI: 10.3390/molecules23092374
Synthesis and Antisense Properties of 2'β-F-Arabinouridine Modified Oligonucleotides with 4'- C-OMe Substituent
Abstract
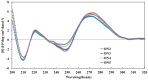
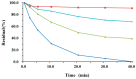
A novel 2'-F,4'-C-OMe⁻arabinouridine (araU) was successfully synthesized and introduced into oligonucleotides. The oligonucleotide containing 2'-F,4'-C-OMe⁻araU exhibited improved nuclease resistance and RNA hybridizing selective ability relative to 2'-F⁻araU. In particular, when 2'-F,4'-C-OMe⁻araU inserted into C⁻H⋯F⁻C bonding-favorable 5'⁻uridine⁻purine⁻3' steps, the modified oligonucleotide showed remarkable binding affinity and selectivity to RNA complements. Thus, 2'-F,4'-C-OMe⁻araU has valuable antisense properties and can be used as novel chemical modification for antisense therapeutic strategy.
Keywords: arabinonucleotide; chemical modification; fluorine; pseudohydrogen bond.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
[Synthesis and antisense properties of 2’β-F- and 2’α-F-2’-deoxy-uridines modified oligonucleotides with 4’-C-(2-methoxyethoxy) substituent].Yao Xue Xue Bao. 2016 Aug;51(8):1271-80. Yao Xue Xue Bao. 2016. PMID: 29905991 Chinese.
-
Synthesis of 2'-O-[2-[(N,N-dimethylamino)oxy]ethyl] modified nucleosides and oligonucleotides.J Org Chem. 2002 Jan 25;67(2):357-69. doi: 10.1021/jo0103975. J Org Chem. 2002. PMID: 11798305
-
2'-O-[2-[2-(N,N-dimethylamino)ethoxy]ethyl] modified oligonucleotides: symbiosis of charge interaction factors and stereoelectronic effects.Org Lett. 2003 Jun 12;5(12):2017-20. doi: 10.1021/ol0340991. Org Lett. 2003. PMID: 12790517
-
An overview of sugar-modified oligonucleotides for antisense therapeutics.Chem Biodivers. 2011 Sep;8(9):1616-41. doi: 10.1002/cbdv.201100081. Chem Biodivers. 2011. PMID: 21922654 Review.
-
Chemical modification and design of anti-miRNA oligonucleotides.Gene Ther. 2011 Dec;18(12):1111-20. doi: 10.1038/gt.2011.100. Epub 2011 Jul 14. Gene Ther. 2011. PMID: 21753793 Review.
Cited by
-
Nanomedicine for Gene Delivery and Drug Repurposing in the Treatment of Muscular Dystrophies.Pharmaceutics. 2021 Feb 19;13(2):278. doi: 10.3390/pharmaceutics13020278. Pharmaceutics. 2021. PMID: 33669654 Free PMC article. Review.
-
Nanomedicine for Treating Muscle Dystrophies: Opportunities, Challenges, and Future Perspectives.Int J Mol Sci. 2022 Oct 10;23(19):12039. doi: 10.3390/ijms231912039. Int J Mol Sci. 2022. PMID: 36233338 Free PMC article. Review.
-
Effect of Sugar 2',4'-Modifications on Gene Silencing Activity of siRNA Duplexes.Nucleic Acid Ther. 2019 Aug;29(4):187-194. doi: 10.1089/nat.2019.0792. Epub 2019 May 14. Nucleic Acid Ther. 2019. PMID: 31084536 Free PMC article.
-
Specificity of oligonucleotide gene therapy (OGT) agents.Theranostics. 2022 Oct 9;12(16):7132-7157. doi: 10.7150/thno.77830. eCollection 2022. Theranostics. 2022. PMID: 36276652 Free PMC article. Review.
References
-
- Deleavey G.F., Damha M.J. Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 2012;19:937–954. - PubMed
-
- Ellipilli S., Ganesh K.N. Fluorous peptide nucleic acids: PNA analogues with fluorine in backbone (γ-CF2-apg-PNA) enhance cellular uptake. J. Org. Chem. 2015;80:9185–9191. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

