Synthesis and Antisense Properties of 2'β-F-Arabinouridine Modified Oligonucleotides with 4'- C-OMe Substituent
- PMID: 30227644
- PMCID: PMC6225415
- DOI: 10.3390/molecules23092374
Synthesis and Antisense Properties of 2'β-F-Arabinouridine Modified Oligonucleotides with 4'- C-OMe Substituent
Abstract
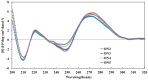
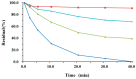
A novel 2'-F,4'-C-OMe⁻arabinouridine (araU) was successfully synthesized and introduced into oligonucleotides. The oligonucleotide containing 2'-F,4'-C-OMe⁻araU exhibited improved nuclease resistance and RNA hybridizing selective ability relative to 2'-F⁻araU. In particular, when 2'-F,4'-C-OMe⁻araU inserted into C⁻H⋯F⁻C bonding-favorable 5'⁻uridine⁻purine⁻3' steps, the modified oligonucleotide showed remarkable binding affinity and selectivity to RNA complements. Thus, 2'-F,4'-C-OMe⁻araU has valuable antisense properties and can be used as novel chemical modification for antisense therapeutic strategy.
Keywords: arabinonucleotide; chemical modification; fluorine; pseudohydrogen bond.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Deleavey G.F., Damha M.J. Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 2012;19:937–954. - PubMed
-
- Ellipilli S., Ganesh K.N. Fluorous peptide nucleic acids: PNA analogues with fluorine in backbone (γ-CF2-apg-PNA) enhance cellular uptake. J. Org. Chem. 2015;80:9185–9191. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

