Extracellular matrix collagen I promotes the tumor progression of residual hepatocellular carcinoma after heat treatment
- PMID: 30227844
- PMCID: PMC6145107
- DOI: 10.1186/s12885-018-4820-9
Extracellular matrix collagen I promotes the tumor progression of residual hepatocellular carcinoma after heat treatment
Abstract
Background: Accelerated malignant behaviors induced by insufficient thermal ablation have been increasingly reported, however, the exact mechanisms are still unclear. Here, we investigated the importance of the extracellular matrix (ECM) in modulating the progression of residual hepatocellular carcinoma (HCC) after heat treatment.
Methods: Heat-exposed residual HCC cells were cultured in different ECM gels. We used basement membrane gel (Matrigel) to simulate the normal microenvironment and collagen I to model the pathological stromal ECM. The alterations of morphology and parameters of proliferation, epithelial-mesenchymal transition (EMT) and stemness were analyzed in vitro and in vivo.
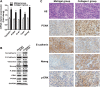
Results: Increased collagen I deposition was observed at the periablational zone after incomplete RFA of HCC in a xenograft model. The markers of cell proliferation, EMT, motility and progenitor-like traits of heat-exposed residual HCC cells were significantly induced by collagen I as compared to Matrigel (p values all < 0.05). Importantly, collagen I induced the activation of ERK phosphorylation in heat-exposed residual HCC cells. ERK1/2 inhibitor reversed the collagen I-promoted ERK phosphorylation, cell proliferative, protrusive and spindle-like appearance of heat-treated residual HCC cells in vitro. Moreover, collagen I promoted the in vivo tumor progression of heat-exposed residual HCC cells, and sorafenib markedly reversed the collagen I-mediated protumor effects.
Conclusions: Our findings demonstrate that collagen I could enhance the aggressive progression of residual HCC cells after suboptimal heat treatment and sorafenib may be a treatment approach to thwart this process.
Keywords: Collagen I; ERK; Heat treatment; Hepatocellular carcinoma.
Conflict of interest statement
Ethics approval
All animal experiments were carried out in compliance with the guidelines by the Shanghai Medical Experimental Animal Care Commission. The experimental protocols were approved by the Ethical Committee on Animal Experiments of Fudan University, Shanghai China (Permit Number: 201807002Z).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

