Contraception after Abortion and Risk of Repeated Unintended Pregnancy among Health Plan Members
- PMID: 30227910
- PMCID: PMC6141650
- DOI: 10.7812/TPP/18-058
Contraception after Abortion and Risk of Repeated Unintended Pregnancy among Health Plan Members
Abstract
Context: Optimizing access to effective contraception at the time of abortion can reduce repeated unintended pregnancies.
Objective: To assess contraception initiation and repeated unintended pregnancies among women receiving abortions in Kaiser Permanente Northern California (KPNC) facilities and through outside contracted facilities.
Design: A retrospective cohort study was conducted using a randomized proportional sample of women aged 15 to 44 years having abortions in KPNC, to determine contraception initiation within 90 days. Demographic and clinical characteristics (age, race/ethnicity, gravidity, parity, contraceptive method initiated, and pregnancies within 12 months) were collected from electronic health records. Descriptive statistics, χ2 tests, t-tests, and logistic regression models assessed predictors of long-acting reversible contraception (LARC) initiation and having another unintended pregnancy within 12 months of abortion.
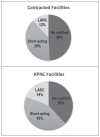
Results: Women having abortions from contracted facilities were significantly less likely to initiate LARC within 90 days compared with those receiving abortions in KPNC facilities (11.99% vs 19.10%, p = 0.012). Significant factors associated with 90-day LARC initiation included abortions in KPNC facilities (adjusted odds ratio [aOR] = 1.87, p = 0.007) and gravidity of 3 or more. Women initiating short-acting or no contraception were significantly more likely to have an unintended pregnancy within 12 months of the abortion than those initiating LARC (aOR = 3.66, p = 0.005; no contraception vs LARC, aOR = 3.75, p = 0.005).
Conclusion: In response to this study, KPNC now provides reimbursement for LARC in all outside abortion contracts, internalized more abortions in KPNC facilities, and strengthened clinical recommendations for immediate, effective postabortion contraception, especially LARC.
Conflict of interest statement
Four of the authors (Debbie Postlethwaite, Maqdooda Merchant, Amy Alabaster, and Tina Raine-Bennett) are participating in a US Food and Drug Administration-mandated safety study funded by Bayer Global. The author(s) have no additional conflicts of interest to disclose.
Figures
Similar articles
-
Are Health Plan Design and Prior Use of Long-Acting Reversible Contraception Associated with Pregnancy Intention?J Womens Health (Larchmt). 2017 May;26(5):450-460. doi: 10.1089/jwh.2014.5146. Epub 2016 Oct 18. J Womens Health (Larchmt). 2017. PMID: 27753522
-
Risk factors and the choice of long-acting reversible contraception following medical abortion: effect on subsequent induced abortion and unwanted pregnancy.Eur J Contracept Reprod Health Care. 2018 Apr;23(2):89-96. doi: 10.1080/13625187.2018.1440385. Epub 2018 Mar 14. Eur J Contracept Reprod Health Care. 2018. PMID: 29537321
-
Factors associated with short interpregnancy interval in women who plan postpartum LARC: a retrospective study.Contraception. 2017 Mar;95(3):245-250. doi: 10.1016/j.contraception.2016.08.012. Epub 2016 Aug 30. Contraception. 2017. PMID: 27589883
-
Long-acting reversible contraception: a practical solution to reduce unintended pregnancy.Minerva Ginecol. 2013 Jun;65(3):271-7. Minerva Ginecol. 2013. PMID: 23689169 Review.
-
The complex relationship between contraception and abortion.Best Pract Res Clin Obstet Gynaecol. 2020 Jan;62:90-100. doi: 10.1016/j.bpobgyn.2019.04.007. Epub 2019 May 6. Best Pract Res Clin Obstet Gynaecol. 2020. PMID: 31196674 Review.
Cited by
-
Prevalence and Predictors of Contraceptives Use among Women Aged (15-49 years) with Induced Abortion History in Ghana.Adv Prev Med. 2020 Aug 28;2020:2630905. doi: 10.1155/2020/2630905. eCollection 2020. Adv Prev Med. 2020. PMID: 32908708 Free PMC article.
References
-
- Hohmann H, Reeves MF, Chen BA, Perriera LK, Hayes JL, Creinin MD. Immediate versus delayed insertion of the levonorgestral-releasing intrauterine device following dilation and evacuation: A randomized controlled trial. Contraception. 2012 Mar;85(3):240–5. doi: 10.1016/j.contraception.2009.05.058. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical