A Practical Guide for Treatment of Rapidly Progressive ADPKD with Tolvaptan
- PMID: 30228150
- PMCID: PMC6171265
- DOI: 10.1681/ASN.2018060590
A Practical Guide for Treatment of Rapidly Progressive ADPKD with Tolvaptan
Abstract
In the past, the treatment of autosomal dominant polycystic kidney disease (ADPKD) has been limited to the management of its symptoms and complications. Recently, the US Food and Drug Administration (FDA) approved tolvaptan as the first drug treatment to slow kidney function decline in adults at risk of rapidly progressing ADPKD. Full prescribing information approved by the FDA provides helpful guidelines but does not address practical questions that are being raised by nephrologists, internists, and general practitioners taking care of patients with ADPKD, and by the patients themselves. In this review, we provide practical guidance and discuss steps that require consideration before and after prescribing tolvaptan to patients with ADPKD to ensure that this treatment is implemented safely and effectively. These steps include confirmation of diagnosis; identification of rapidly progressive disease; implementation of basic renal protective measures; counseling of patients on potential benefits and harms; exclusions to use; education of patients on aquaresis and its expected consequences; initiation, titration, and optimization of tolvaptan treatment; prevention of aquaresis-related complications; evaluation and management of liver enzyme elevations; and monitoring of treatment efficacy. Our recommendations are made on the basis of published evidence and our collective experiences during the randomized, clinical trials and open-label extension studies of tolvaptan in ADPKD.
Keywords: ADPKD; Tolvaptan; V2 Receptor Antagonist; hepatotoxicity; polycystic kidney disease; vasopressin.
Copyright © 2018 by the American Society of Nephrology.
Figures

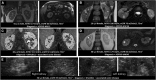
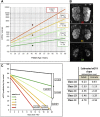
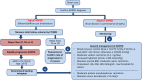
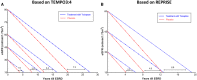
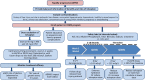
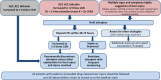
Comment in
-
Tolvaptan or transplant: why wait?Kidney Int. 2020 Aug;98(2):286-289. doi: 10.1016/j.kint.2020.03.022. Kidney Int. 2020. PMID: 32709289 No abstract available.
References
-
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al. : REPRISE Trial Investigators: Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377: 1930–1942, 2017 - PubMed
-
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Dandurand A, et al. : TEMPO 4:4 Trial Investigators: Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: The TEMPO 4:4 Trial. Nephrol Dial Transplant 32: 1262, 2017 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

