Occluding junctions as novel regulators of tissue mechanics during wound repair
- PMID: 30228162
- PMCID: PMC6279375
- DOI: 10.1083/jcb.201804048
Occluding junctions as novel regulators of tissue mechanics during wound repair
Abstract
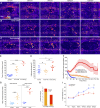
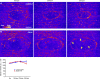
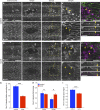
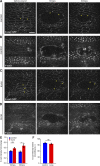
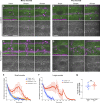
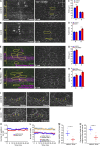
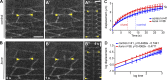
In epithelial tissues, cells tightly connect to each other through cell-cell junctions, but they also present the remarkable capacity of reorganizing themselves without compromising tissue integrity. Upon injury, simple epithelia efficiently resolve small lesions through the action of actin cytoskeleton contractile structures at the wound edge and cellular rearrangements. However, the underlying mechanisms and how they cooperate are still poorly understood. In this study, we combine live imaging and theoretical modeling to reveal a novel and indispensable role for occluding junctions (OJs) in this process. We demonstrate that OJ loss of function leads to defects in wound-closure dynamics: instead of contracting, wounds dramatically increase their area. OJ mutants exhibit phenotypes in cell shape, cellular rearrangements, and mechanical properties as well as in actin cytoskeleton dynamics at the wound edge. We propose that OJs are essential for wound closure by impacting on epithelial mechanics at the tissue level, which in turn is crucial for correct regulation of the cellular events occurring at the wound edge.
© 2018 Carvalho et al.
Figures

Similar articles
-
Dachsous is a key player in epithelial wound closure through modulating cell shape changes and tissue mechanics.J Cell Sci. 2025 Mar 1;138(5):JCS263674. doi: 10.1242/jcs.263674. Epub 2025 Mar 12. J Cell Sci. 2025. PMID: 39945479
-
Endocytosis-dependent coordination of multiple actin regulators is required for wound healing.J Cell Biol. 2015 Aug 3;210(3):419-33. doi: 10.1083/jcb.201411037. Epub 2015 Jul 27. J Cell Biol. 2015. PMID: 26216900 Free PMC article.
-
Bicellular Tight Junctions and Wound Healing.Int J Mol Sci. 2018 Dec 4;19(12):3862. doi: 10.3390/ijms19123862. Int J Mol Sci. 2018. PMID: 30518037 Free PMC article. Review.
-
Recapitulation of morphogenetic cell shape changes enables wound re-epithelialisation.Development. 2014 May;141(9):1814-20. doi: 10.1242/dev.107045. Epub 2014 Apr 9. Development. 2014. PMID: 24718989 Free PMC article.
-
Tension (re)builds: Biophysical mechanisms of embryonic wound repair.Mech Dev. 2017 Apr;144(Pt A):43-52. doi: 10.1016/j.mod.2016.11.004. Epub 2016 Dec 15. Mech Dev. 2017. PMID: 27989746 Review.
Cited by
-
Polarity during tissue repair, a multiscale problem.Curr Opin Cell Biol. 2020 Feb;62:31-36. doi: 10.1016/j.ceb.2019.07.015. Epub 2019 Sep 9. Curr Opin Cell Biol. 2020. PMID: 31514044 Free PMC article. Review.
-
Forceful closure: cytoskeletal networks in embryonic wound repair.Mol Biol Cell. 2019 Jun 1;30(12):1353-1358. doi: 10.1091/mbc.E18-04-0248. Mol Biol Cell. 2019. PMID: 31145669 Free PMC article. Review.
-
A role for Myo-II zipper and spaghetti squash in Gliotactin-dependent Drosophila melanogaster wing hair planar cell polarity.PLoS One. 2025 Jul 23;20(7):e0328970. doi: 10.1371/journal.pone.0328970. eCollection 2025. PLoS One. 2025. PMID: 40700419 Free PMC article.
-
Casein kinase 1α decreases β-catenin levels at adherens junctions to facilitate wound closure in Drosophila larvae.Development. 2019 Oct 2;146(20):dev175133. doi: 10.1242/dev.175133. Development. 2019. PMID: 31511254 Free PMC article.
-
Atypical septate junctions maintain the somatic enclosure around maturing spermatids and prevent premature sperm release in Drosophila testis.Biol Open. 2019 Feb 4;8(2):bio036939. doi: 10.1242/bio.036939. Biol Open. 2019. PMID: 30635267 Free PMC article.
References
-
- Brand A.H., and Perrimon N.. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118:401–415. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

