K-Ras Lys-42 is crucial for its signaling, cell migration, and invasion
- PMID: 30228186
- PMCID: PMC6231119
- DOI: 10.1074/jbc.RA118.003723
K-Ras Lys-42 is crucial for its signaling, cell migration, and invasion
Abstract
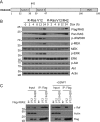
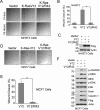
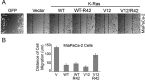
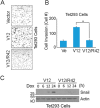
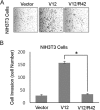
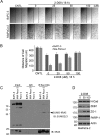
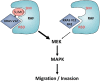
Ras proteins participate in multiple signal cascades, regulating crucial cellular processes, including cell survival, proliferation, and differentiation. We have previously reported that Ras proteins are modified by sumoylation and that Lys-42 plays an important role in mediating the modification. In the current study, we further investigated the role of Lys-42 in regulating cellular activities of K-Ras. Inducible expression of K-RasV12 led to the activation of downstream components, including c-RAF, MEK1, and extracellular signal-regulated kinases (ERKs), whereas expression of K-RasV12/R42 mutant compromised the activation of the RAF/MEK/ERK signaling axis. Expression of K-RasV12/R42 also led to reduced phosphorylation of several other protein kinases, including c-Jun N-terminal kinase (JNK), Chk2, and focal adhesion kinase (FAK). Significantly, K-RasV12/R42 expression inhibited cellular migration and invasion in vitro in multiple cell lines, including transformed pancreatic cells. Given that K-Ras plays a crucial role in mediating oncogenesis in the pancreas, we treated transformed pancreatic cells of both BxPC-3 and MiaPaCa-2 with 2-D08, a small ubiquitin-like modifier (SUMO) E2 inhibitor. Treatment with the compound inhibited cell migration in a concentration-dependent manner, which was correlated with a reduced level of K-Ras sumoylation. Moreover, 2-D08 suppressed expression of ZEB1 (a mesenchymal cell marker) with concomitant induction of ZO-1 (an epithelial cell marker). Combined, our studies strongly suggest that posttranslational modification(s), including sumoylation mediated by Lys-42, plays a crucial role in K-Ras activities in vivo.
Keywords: K-Ras; Ras protein; cell invasion; cell migration; domain structure; lysine residue; posttranslational modification (PTM); signaling; sumoylation; transformation.
© 2018 Choi et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
Ras sumoylation in cell signaling and transformation.Semin Cancer Biol. 2021 Nov;76:301-309. doi: 10.1016/j.semcancer.2021.03.033. Epub 2021 Apr 1. Semin Cancer Biol. 2021. PMID: 33812985 Review.
-
SUMOylation of Grb2 enhances the ERK activity by increasing its binding with Sos1.Mol Cancer. 2014 Apr 29;13:95. doi: 10.1186/1476-4598-13-95. Mol Cancer. 2014. PMID: 24775912 Free PMC article.
-
Small G proteins Rac1 and Ras regulate serine/threonine protein phosphatase 5 (PP5)·extracellular signal-regulated kinase (ERK) complexes involved in the feedback regulation of Raf1.J Biol Chem. 2014 Feb 14;289(7):4219-32. doi: 10.1074/jbc.M113.518514. Epub 2013 Dec 26. J Biol Chem. 2014. PMID: 24371145 Free PMC article.
-
Rasfonin, a novel 2-pyrone derivative, induces ras-mutated Panc-1 pancreatic tumor cell death in nude mice.Cell Death Dis. 2014 May 22;5(5):e1241. doi: 10.1038/cddis.2014.213. Cell Death Dis. 2014. PMID: 24853419 Free PMC article.
-
ERK signaling for cell migration and invasion.Front Mol Biosci. 2022 Oct 3;9:998475. doi: 10.3389/fmolb.2022.998475. eCollection 2022. Front Mol Biosci. 2022. PMID: 36262472 Free PMC article. Review.
Cited by
-
Post-translational modification of RAS proteins.Curr Opin Struct Biol. 2021 Dec;71:180-192. doi: 10.1016/j.sbi.2021.06.015. Epub 2021 Aug 6. Curr Opin Struct Biol. 2021. PMID: 34365229 Free PMC article. Review.
-
Oncogenic Kras-Mediated Cytokine CCL15 Regulates Pancreatic Cancer Cell Migration and Invasion through ROS.Cancers (Basel). 2022 Apr 26;14(9):2153. doi: 10.3390/cancers14092153. Cancers (Basel). 2022. PMID: 35565279 Free PMC article.
-
2-D08 treatment regulates C2C12 myoblast proliferation and differentiation via the Erk1/2 and proteasome signaling pathways.J Muscle Res Cell Motil. 2021 Jun;42(2):193-202. doi: 10.1007/s10974-021-09605-x. Epub 2021 Jun 17. J Muscle Res Cell Motil. 2021. PMID: 34142311 Free PMC article.
-
2‑D08 mediates notable anticancer effects through multiple cellular pathways in uterine leiomyosarcoma cells.Oncol Rep. 2024 Jul;52(1):97. doi: 10.3892/or.2024.8756. Epub 2024 Jun 14. Oncol Rep. 2024. PMID: 38874019 Free PMC article.
-
Rac1 GTPase Regulates the βTrCP-Mediated Proteolysis of YAP Independently of the LATS1/2 Kinases.Cancers (Basel). 2024 Oct 25;16(21):3605. doi: 10.3390/cancers16213605. Cancers (Basel). 2024. PMID: 39518045 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

