CREST in the Nucleus Accumbens Core Regulates Cocaine Conditioned Place Preference, Cocaine-Seeking Behavior, and Synaptic Plasticity
- PMID: 30228227
- PMCID: PMC6209848
- DOI: 10.1523/JNEUROSCI.2911-17.2018
CREST in the Nucleus Accumbens Core Regulates Cocaine Conditioned Place Preference, Cocaine-Seeking Behavior, and Synaptic Plasticity
Abstract
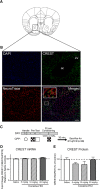
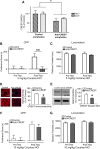
Epigenetic mechanisms result in persistent changes at the cellular level that can lead to long-lasting behavioral adaptations. Nucleosome remodeling is a major epigenetic mechanism that has not been well explored with regards to drug-seeking behaviors. Nucleosome remodeling is performed by multi-subunit complexes that interact with DNA or chromatin structure and possess an ATP-dependent enzyme to disrupt nucleosome-DNA contacts and ultimately regulate gene expression. Calcium responsive transactivator (CREST) is a transcriptional activator that interacts with enzymes involved in both histone acetylation and nucleosome remodeling. Here, we examined the effects of knocking down CREST in the nucleus accumbens (NAc) core on drug-seeking behavior and synaptic plasticity in male mice as well as drug-seeking in male rats. Knocking down CREST in the NAc core results in impaired cocaine-induced conditioned place preference (CPP) as well as theta-induced long-term potentiation in the NAc core. Further, similar to the CPP findings, using a self-administration procedure, we found that CREST knockdown in the NAc core of male rats had no effect on instrumental responding for cocaine itself on a first-order schedule, but did significantly attenuate responding on a second-order chain schedule, in which responding has a weaker association with cocaine. Together, these results suggest that CREST in the NAc core is required for cocaine-induced CPP, synaptic plasticity, as well as cocaine-seeking behavior.SIGNIFICANCE STATEMENT This study demonstrates a key role for the role of Calcium responsive transactivator (CREST), a transcriptional activator, in the nucleus accumbens (NAc) core with regard to cocaine-induced conditioned place preference (CPP), self-administration (SA), and synaptic plasticity. CREST is a unique transcriptional regulator that can recruit enzymes from two different major epigenetic mechanisms: histone acetylation and nucleosome remodeling. In this study we also found that the level of potentiation in the NAc core correlated with whether or not animals formed a CPP. Together the results indicate that CREST is a key downstream regulator of cocaine action in the NAc.
Keywords: CREST; LTP; cocaine; epigenetics; nucleosome remodeling; nucleus accumbens.
Copyright © 2018 the authors 0270-6474/18/389515-13$15.00/0.
Figures




Similar articles
-
HDAC3 Activity within the Nucleus Accumbens Regulates Cocaine-Induced Plasticity and Behavior in a Cell-Type-Specific Manner.J Neurosci. 2021 Mar 31;41(13):2814-2827. doi: 10.1523/JNEUROSCI.2829-20.2021. Epub 2021 Feb 18. J Neurosci. 2021. PMID: 33602824 Free PMC article.
-
CaMKII activity in the ventral tegmental area gates cocaine-induced synaptic plasticity in the nucleus accumbens.Neuropsychopharmacology. 2014 Mar;39(4):989-99. doi: 10.1038/npp.2013.299. Epub 2013 Oct 24. Neuropsychopharmacology. 2014. PMID: 24154664 Free PMC article.
-
Dnmt3a2 in the Nucleus Accumbens Shell Is Required for Reinstatement of Cocaine Seeking.J Neurosci. 2018 Aug 22;38(34):7516-7528. doi: 10.1523/JNEUROSCI.0600-18.2018. Epub 2018 Jul 20. J Neurosci. 2018. PMID: 30030395 Free PMC article.
-
Targeting Neuroplasticity in Substance Use Disorders: Implications for Therapeutics.Annu Rev Pharmacol Toxicol. 2025 Jan;65(1):259-280. doi: 10.1146/annurev-pharmtox-061724-080548. Epub 2024 Dec 17. Annu Rev Pharmacol Toxicol. 2025. PMID: 39374445 Review.
-
Cocaine-induced homeostatic regulation and dysregulation of nucleus accumbens neurons.Behav Brain Res. 2011 Jan 1;216(1):9-18. doi: 10.1016/j.bbr.2010.07.039. Epub 2010 Aug 11. Behav Brain Res. 2011. PMID: 20708038 Free PMC article. Review.
Cited by
-
Individual differences in cocaine-induced conditioned place preference in male rats: Behavioral and transcriptomic evidence.J Psychopharmacol. 2022 Oct;36(10):1161-1175. doi: 10.1177/02698811221123047. Epub 2022 Sep 19. J Psychopharmacol. 2022. PMID: 36121009 Free PMC article.
-
Context-Dependent and Context-Independent Effects of D1 Receptor Antagonism in the Basolateral and Central Amygdala during Cocaine Self-Administration.eNeuro. 2019 Aug 13;6(4):ENEURO.0203-19.2019. doi: 10.1523/ENEURO.0203-19.2019. Print 2019 Jul/Aug. eNeuro. 2019. PMID: 31358512 Free PMC article.
-
HDAC3-Mediated Repression of the Nr4a Family Contributes to Age-Related Impairments in Long-Term Memory.J Neurosci. 2019 Jun 19;39(25):4999-5009. doi: 10.1523/JNEUROSCI.2799-18.2019. Epub 2019 Apr 18. J Neurosci. 2019. PMID: 31000586 Free PMC article.
-
The effect of the mGlu8 receptor agonist, (S)-3,4-DCPG on acquisition and expression of morphine-induced conditioned place preference in male rats.Behav Brain Funct. 2021 Feb 21;17(1):1. doi: 10.1186/s12993-021-00174-0. Behav Brain Funct. 2021. PMID: 33612106 Free PMC article.
-
HDAC3 Activity within the Nucleus Accumbens Regulates Cocaine-Induced Plasticity and Behavior in a Cell-Type-Specific Manner.J Neurosci. 2021 Mar 31;41(13):2814-2827. doi: 10.1523/JNEUROSCI.2829-20.2021. Epub 2021 Feb 18. J Neurosci. 2021. PMID: 33602824 Free PMC article.
References
-
- Alaghband Y, Kwapis JL, López AJ, White AO, Aimiuwu OV, Al-Kachak A, Bodinayake KK, Oparaugo NC, Dang R, Astarabadi M, Matheos DP, Wood MA (2017) Distinct roles for the deacetylase domain of HDAC3 in the hippocampus and medial prefrontal cortex in the formation and extinction of memory. Neurobiol Learn Mem 145:94–104. 10.1016/j.nlm.2017.09.001 - DOI - PMC - PubMed
-
- Chesi A, Staahl BT, Jovičić A, Couthouis J, Fasolino M, Raphael AR, Yamazaki T, Elias L, Polak M, Kelly C, Williams KL, Fifita JA, Maragakis NJ, Nicholson GA, King OD, Reed R, Crabtree GR, Blair IP, Glass JD, Gitler AD (2013) Exome sequencing to identify de novo mutations in sporadic ALS trios. Nat Neurosci 16:851–855. 10.1038/nn.3412 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
