Neogenin in Amygdala for Neuronal Activity and Information Processing
- PMID: 30228230
- PMCID: PMC6209834
- DOI: 10.1523/JNEUROSCI.0433-18.2018
Neogenin in Amygdala for Neuronal Activity and Information Processing
Abstract
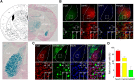
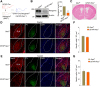
Fear learning and memory are vital for livings to survive, dysfunctions in which have been implicated in various neuropsychiatric disorders. Appropriate neuronal activation in amygdala is critical for fear memory. However, the underlying regulatory mechanisms are not well understood. Here we report that Neogenin, a DCC (deleted in colorectal cancer) family receptor, which plays important roles in axon navigation and adult neurogenesis, is enriched in excitatory neurons in BLA (Basolateral amygdala). Fear memory is impaired in male Neogenin mutant mice. The number of cFos+ neurons in response to tone-cued fear training was reduced in mutant mice, indicating aberrant neuronal activation in the absence of Neogenin. Electrophysiological studies show that Neogenin mutation reduced the cortical afferent input to BLA pyramidal neurons and compromised both induction and maintenance of Long-Term Potentiation evoked by stimulating cortical afferent, suggesting a role of Neogenin in synaptic plasticity. Concomitantly, there was a reduction in spine density and in frequency of miniature excitatory postsynaptic currents (mEPSCs), but not miniature inhibitory postsynaptic currents, suggesting a role of Neogenin in forming excitatory synapses. Finally, ablating Neogenin in the BLA in adult male mice impaired fear memory likely by reducing mEPSC frequency in BLA excitatory neurons. These results reveal an unrecognized function of Neogenin in amygdala for information processing by promoting and maintaining neurotransmission and synaptic plasticity and provide insight into molecular mechanisms of neuronal activation in amygdala.SIGNIFICANCE STATEMENT Appropriate neuronal activation in amygdala is critical for information processing. However, the underlying regulatory mechanisms are not well understood. Neogenin is known to regulate axon navigation and adult neurogenesis. Here we show that it is critical for neurotransmission and synaptic plasticity in the amygdala and thus fear memory by using a combination of genetic, electrophysiological, behavioral techniques. Our studies identify a novel function of Neogenin and provide insight into molecular mechanisms of neuronal activation in amygdala for fear processing.
Keywords: Neogenin; amygdala; fear memory; synaptic transmission.
Copyright © 2018 the authors 0270-6474/18/389600-14$15.00/0.
Figures






Similar articles
-
Deficiency of the CYLD Impairs Fear Memory of Mice and Disrupts Neuronal Activity and Synaptic Transmission in the Basolateral Amygdala.Front Cell Neurosci. 2021 Sep 17;15:740165. doi: 10.3389/fncel.2021.740165. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34602983 Free PMC article.
-
GABAergic Synapses at the Axon Initial Segment of Basolateral Amygdala Projection Neurons Modulate Fear Extinction.Neuropsychopharmacology. 2017 Jan;42(2):473-484. doi: 10.1038/npp.2016.205. Epub 2016 Sep 16. Neuropsychopharmacology. 2017. PMID: 27634356 Free PMC article.
-
ASIC-dependent LTP at multiple glutamatergic synapses in amygdala network is required for fear memory.Sci Rep. 2015 May 19;5:10143. doi: 10.1038/srep10143. Sci Rep. 2015. PMID: 25988357 Free PMC article.
-
GABAergic interneurons: The orchestra or the conductor in fear learning and memory?Brain Res Bull. 2018 Jul;141:13-19. doi: 10.1016/j.brainresbull.2017.11.016. Epub 2017 Dec 2. Brain Res Bull. 2018. PMID: 29197563 Free PMC article. Review.
-
Fear conditioning and the basolateral amygdala.F1000Res. 2020 Jan 28;9:F1000 Faculty Rev-53. doi: 10.12688/f1000research.21201.1. eCollection 2020. F1000Res. 2020. PMID: 32047613 Free PMC article. Review.
Cited by
-
Hippocampal astrocytic neogenin regulating glutamate uptake, a critical pathway for preventing epileptic response.Proc Natl Acad Sci U S A. 2021 Apr 20;118(16):e2022921118. doi: 10.1073/pnas.2022921118. Proc Natl Acad Sci U S A. 2021. PMID: 33850017 Free PMC article.
-
T-Cell Immunotherapy for Pediatric High-Grade Gliomas: New Insights to Overcoming Therapeutic Challenges.Front Oncol. 2021 Oct 25;11:718030. doi: 10.3389/fonc.2021.718030. eCollection 2021. Front Oncol. 2021. PMID: 34760690 Free PMC article. Review.
-
Neuregulin 1/ErbB4 signaling contributes to the anti-epileptic effects of the ketogenic diet.Cell Biosci. 2021 Feb 3;11(1):29. doi: 10.1186/s13578-021-00536-1. Cell Biosci. 2021. PMID: 33536056 Free PMC article.
-
Social Company by a Receptive Mating Partner Facilitates Fear Extinction.Front Neurosci. 2020 Feb 7;14:62. doi: 10.3389/fnins.2020.00062. eCollection 2020. Front Neurosci. 2020. PMID: 32116509 Free PMC article.
-
Awakening the dormant: Role of axonal guidance cues in stress-induced reorganization of the adult prefrontal cortex leading to depression-like behavior.Front Neural Circuits. 2023 Mar 24;17:1113023. doi: 10.3389/fncir.2023.1113023. eCollection 2023. Front Neural Circuits. 2023. PMID: 37035502 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
