Olfactory receptor OR2AT4 regulates human hair growth
- PMID: 30228264
- PMCID: PMC6143528
- DOI: 10.1038/s41467-018-05973-0
Olfactory receptor OR2AT4 regulates human hair growth
Abstract
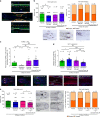
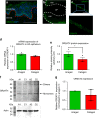
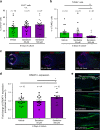
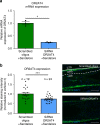
Olfactory receptors are expressed by different cell types throughout the body and regulate physiological cell functions beyond olfaction. In particular, the olfactory receptor OR2AT4 has been shown to stimulate keratinocyte proliferation in the skin. Here, we show that the epithelium of human hair follicles, particularly the outer root sheath, expresses OR2AT4, and that specific stimulation of OR2AT4 by a synthetic sandalwood odorant (Sandalore®) prolongs human hair growth ex vivo by decreasing apoptosis and increasing production of the anagen-prolonging growth factor IGF-1. In contrast, co-administration of the specific OR2AT4 antagonist Phenirat® and silencing of OR2AT4 inhibit hair growth. Together, our study identifies that human hair follicles can engage in olfactory receptor-dependent chemosensation and require OR2AT4-mediated signaling to sustain their growth, suggesting that olfactory receptors may serve as a target in hair loss therapy.
Conflict of interest statement
J.C., M.B., L.P., J.L. and M.A. are or were employees of Monasterium Laboratory GmbH, Münster, which was founded by R.P. R.P., also serves as consultant for Giuliani Pharma, which has filed a patent on the use of compounds and compositions targeting OR2AT4 for hair growth-promotion or inhibition in humans (wo2017198818 (a1)—compounds for promoting hair growth and/or inhibiting or delaying hair loss in humans, and compositions for such uses). The remaining authors declare no competing interests.
Figures
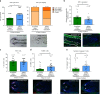
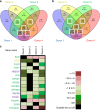

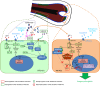
References
-
- Mori, K. (ed) The Olfactory System, 1–18 (Springer, Tokyo, 2014).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

