A high throughput screen for next-generation leads targeting malaria parasite transmission
- PMID: 30228275
- PMCID: PMC6143625
- DOI: 10.1038/s41467-018-05777-2
A high throughput screen for next-generation leads targeting malaria parasite transmission
Abstract
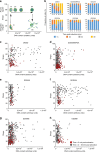
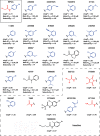
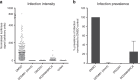
Spread of parasite resistance to artemisinin threatens current frontline antimalarial therapies, highlighting the need for new drugs with alternative modes of action. Since only 0.2-1% of asexual parasites differentiate into sexual, transmission-competent forms, targeting this natural bottleneck provides a tangible route to interrupt disease transmission and mitigate resistance selection. Here we present a high-throughput screen of gametogenesis against a ~70,000 compound diversity library, identifying seventeen drug-like molecules that target transmission. Hit molecules possess varied activity profiles including male-specific, dual acting male-female and dual-asexual-sexual, with one promising N-((4-hydroxychroman-4-yl)methyl)-sulphonamide scaffold found to have sub-micromolar activity in vitro and in vivo efficacy. Development of leads with modes of action focussed on the sexual stages of malaria parasite development provide a previously unexplored base from which future therapeutics can be developed, capable of preventing parasite transmission through the population.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- World Health Organization. World Malaria Report 2017 http://www.who.int/malaria/publications/world-malaria-report-2017/report... (WHO, Geneva, 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

