Cultivated and wild Pleurotus ferulae ethanol extracts inhibit hepatocellular carcinoma cell growth via inducing endoplasmic reticulum stress- and mitochondria-dependent apoptosis
- PMID: 30228276
- PMCID: PMC6143524
- DOI: 10.1038/s41598-018-32225-4
Cultivated and wild Pleurotus ferulae ethanol extracts inhibit hepatocellular carcinoma cell growth via inducing endoplasmic reticulum stress- and mitochondria-dependent apoptosis
Abstract
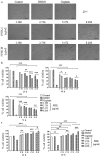
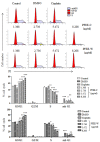
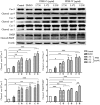
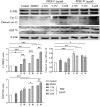
Pleurotus ferulae is a kind of editable mushroom and has various biological functions such as antitumor, antioxidation and immunoregulation. Wild P. ferulae was successfully domesticated but the antitumor function and mechanisms of cultivated and wild P. ferulae need to be compared and explored. Here, we prepared cultivated and wild P. ferulae ethanol extracts (PFEE-C and PFEE-W) and compared their antitumor effect on hepatocellular carcinoma. Our data showed that PFEE-C and PFEE-W significantly inhibited the growth of H22 and HepG2 cells through induction of apoptosis. PFEE-W exhibited higher antitumor activity than PFEE-C. Both PFEE-C and PFEE-W induced endoplasmic reticulum (ER) stress characterized by the up-regulated levels of phosphorylated JNK, cleaved caspase-12 and HSP70, and mitochondrial dysfunction characterized by the reduction of mitochondrial membrane potential and the release of cytochrome c, which promoted the cleavage of caspase-3, -7, -9 and PARP. Moreover, PFEE-C and PFEE-W significantly increased ROS generation in H22 cells and suppressed H22 cell migration through reducing the levels of matrix metalloproteinase -2 and -9. Further, PFEE-C inhibited H22 tumor growth in mouse model and improved the survival of tumor mice. These results indicated that PFEE-C and PFEE-W could inhibit hepatocellular carcinoma cell growth through ER stress- and mitochondria-dependent apoptotic pathways.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Chemical composition, antioxidant and antitumor activities of sub-fractions of wild and cultivated Pleurotus ferulae ethanol extracts.PeerJ. 2018 Dec 20;6:e6097. doi: 10.7717/peerj.6097. eCollection 2018. PeerJ. 2018. PMID: 30595979 Free PMC article.
-
The Extracts of Artemisia absinthium L. Suppress the Growth of Hepatocellular Carcinoma Cells through Induction of Apoptosis via Endoplasmic Reticulum Stress and Mitochondrial-Dependent Pathway.Molecules. 2019 Mar 5;24(5):913. doi: 10.3390/molecules24050913. Molecules. 2019. PMID: 30841648 Free PMC article.
-
16-O-caffeoyl-16-hydroxylhexadecanoic acid, a medicinal plant-derived phenylpropanoid, induces apoptosis in human hepatocarcinoma cells through ROS-dependent endoplasmic reticulum stress.Phytomedicine. 2018 Mar 1;41:33-44. doi: 10.1016/j.phymed.2018.01.024. Epub 2018 Jan 31. Phytomedicine. 2018. PMID: 29519317
-
Ergosterol peroxide from Pleurotus ferulae inhibits gastrointestinal tumor cell growth through induction of apoptosis via reactive oxygen species and endoplasmic reticulum stress.Food Funct. 2020 May 1;11(5):4171-4184. doi: 10.1039/c9fo02454a. Epub 2020 Apr 30. Food Funct. 2020. PMID: 32352095
-
Mitochondrial metabolism: A moving target in hepatocellular carcinoma therapy.J Cell Physiol. 2025 Jan;240(1):e31441. doi: 10.1002/jcp.31441. Epub 2024 Sep 26. J Cell Physiol. 2025. PMID: 39324415 Review.
Cited by
-
Antioxidant Versus Pro-Apoptotic Effects of Mushroom-Enriched Diets on Mitochondria in Liver Disease.Int J Mol Sci. 2019 Aug 16;20(16):3987. doi: 10.3390/ijms20163987. Int J Mol Sci. 2019. PMID: 31426291 Free PMC article. Review.
-
The Emerging Role of Oyster Mushrooms as a Functional Food for Complementary Cancer Therapy.Foods. 2025 Jan 4;14(1):128. doi: 10.3390/foods14010128. Foods. 2025. PMID: 39796417 Free PMC article. Review.
-
Cistanche tubulosa Phenylethanoid Glycosides Induce Apoptosis of Hepatocellular Carcinoma Cells by Mitochondria-Dependent and MAPK Pathways and Enhance Antitumor Effect through Combination with Cisplatin.Integr Cancer Ther. 2021 Jan-Dec;20:15347354211013085. doi: 10.1177/15347354211013085. Integr Cancer Ther. 2021. PMID: 33949239 Free PMC article.
-
Curcumin Sensitizes Kidney Cancer Cells to TRAIL-Induced Apoptosis via ROS Mediated Activation of JNK-CHOP Pathway and Upregulation of DR4.Biology (Basel). 2020 May 1;9(5):92. doi: 10.3390/biology9050092. Biology (Basel). 2020. PMID: 32370057 Free PMC article.
-
Chemical composition, antioxidant and antitumor activities of sub-fractions of wild and cultivated Pleurotus ferulae ethanol extracts.PeerJ. 2018 Dec 20;6:e6097. doi: 10.7717/peerj.6097. eCollection 2018. PeerJ. 2018. PMID: 30595979 Free PMC article.
References
-
- Fitzmaurice C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA oncology. 2017;3:524–548. doi: 10.1001/jamaoncol.2017.1747. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

