Knock-down of AHCY and depletion of adenosine induces DNA damage and cell cycle arrest
- PMID: 30228286
- PMCID: PMC6143609
- DOI: 10.1038/s41598-018-32356-8
Knock-down of AHCY and depletion of adenosine induces DNA damage and cell cycle arrest
Abstract
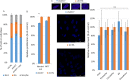
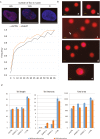
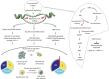
Recently, functional connections between S-adenosylhomocysteine hydrolase (AHCY) activity and cancer have been reported. As the properties of AHCY include the hydrolysis of S-adenosylhomocysteine and maintenance of the cellular methylation potential, the connection between AHCY and cancer is not obvious. The mechanisms by which AHCY influences the cell cycle or cell proliferation have not yet been confirmed. To elucidate AHCY-driven cancer-specific mechanisms, we pursued a multi-omics approach to investigate the effect of AHCY-knockdown on hepatocellular carcinoma cells. Here, we show that reduced AHCY activity causes adenosine depletion with activation of the DNA damage response (DDR), leading to cell cycle arrest, a decreased proliferation rate and DNA damage. The underlying mechanism behind these effects might be applicable to cancer types that have either significant levels of endogenous AHCY and/or are dependent on high concentrations of adenosine in their microenvironments. Thus, adenosine monitoring might be used as a preventive measure in liver disease, whereas induced adenosine depletion might be the desired approach for provoking the DDR in diagnosed cancer, thus opening new avenues for targeted therapy. Additionally, including AHCY in mutational screens as a potential risk factor may be a beneficial preventive measure.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Haba DL, Cantoni G. G.L. The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J. Biol. Chem. 1959;234:603–608. - PubMed
-
- Loehrer FM, Angst CP, Brunner FP, Haefeli WE, Fowler B. Evidence for disturbed S-adenosylmethionine: S-adenosylhomocysteine ratio in patients with end-stage renal failure: a cause for disturbed methylation reactions? Nephrol Dial Transplant. 1998;13:656–661. doi: 10.1093/ndt/13.3.656. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

