Stem cell fate in cancer growth, progression and therapy resistance
- PMID: 30228301
- PMCID: PMC8388042
- DOI: 10.1038/s41568-018-0056-x
Stem cell fate in cancer growth, progression and therapy resistance
Abstract
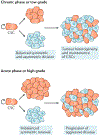
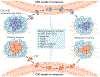
Although we have come a long way in our understanding of the signals that drive cancer growth, and how these signals can be targeted, effective control of this disease remains a key scientific and medical challenge. The therapy resistance and relapse that are commonly seen are driven in large part by the inherent heterogeneity within cancers that allows drugs to effectively eliminate some, but not all, malignant cells. Here, we focus on the fundamental drivers of this heterogeneity by examining emerging evidence that shows that these traits are often controlled by the disruption of normal cell fate and aberrant adoption of stem cell signals. We discuss how undifferentiated cells are preferentially primed for transformation and often serve as the cell of origin for cancers. We also consider evidence showing that activation of stem cell programmes in cancers can lead to progression, therapy resistance and metastatic growth and that targeting these attributes may enable better control over a difficult disease.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
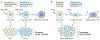
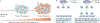


References
-
- Hunger SP, Winick NJ, Sather HN & Carroll WL Therapy of low-risk subsets of childhood acute lymphoblastic leukemia: when do we say enough? Pediatr. Blood Cancer 45, 876–880 (2005). - PubMed
-
- Hodgson DC, Hudson MM & Constine LS Pediatric hodgkin lymphoma: maximizing efficacy and minimizing toxicity. Semin. Radiat. Oncol 17, 230–242 (2007). - PubMed
-
- Barker N et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

