FDA Device Regulation
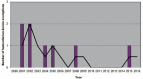
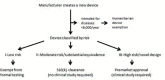
Figures



References
-
- Makower J, Meer A, Denend L. FDA Impact on U.S. Medical Technology Innovation: A Survey of Over 200 Medical Technology Companies. November 2010. 2010 http://advamed.org/res.download/30. Accessed May 28, 2016.
-
- U.S. Food and Drug Administration Overview of Device Regulation. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/d... Accessed March 4, 2017.
-
- Public Citizen. Substantially Unsafe: Medical Devices Pose Great Threat to Patients; Safeguards Must be Strengthened, Not Weakened. https://www.citizen.org/documents/substantially-unsafe-medical-device-re....
-
- Monsein LH. Primer on medical device regulation. Part III. Regulatory mechanisms and import/export regulation. Radiology. 1997;205(1):19–25. - PubMed
-
- Barker JP, Dubin J. Recent Trends in Orthopedic Device Regulation. Orthopedics. 2015;38(7):410–412. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
