MeHg Causes Ultrastructural Changes in Mitochondria and Autophagy in the Spinal Cord Cells of Chicken Embryo
- PMID: 30228816
- PMCID: PMC6136469
- DOI: 10.1155/2018/8460490
MeHg Causes Ultrastructural Changes in Mitochondria and Autophagy in the Spinal Cord Cells of Chicken Embryo
Abstract
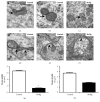
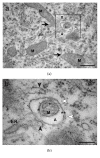
Methylmercury (MeHg) is a known neurodevelopmental toxicant, which causes changes in various structures of the central nervous system (CNS). However, ultrastructural studies of its effects on the developing CNS are still scarce. Here, we investigated the effect of MeHg on the ultrastructure of the cells in spinal cord layers. Chicken embryos at E3 were treated in ovo with 0.1 μg MeHg/50 μL saline solution and analyzed at E10. Then, we used transmission electron microscopy (TEM) to identify possible damage caused by MeHg to the structures and organelles of the spinal cord cells. After MeHg treatment, we observed, in the spinal cord mantle layer, a significant number of altered mitochondria with external membrane disruptions, crest disorganization, swelling in the mitochondrial matrix, and vacuole formation between the internal and external mitochondrial membranes. We also observed dilations in the Golgi complex and endoplasmic reticulum cisterns and the appearance of myelin-like cytoplasmic inclusions. We observed no difference in the total mitochondria number between the control and MeHg-treated groups. However, the MeHg-treated embryos showed an increased number of altered mitochondria and a decreased number of mitochondrial fusion profiles. Additionally, unusual mitochondrial shapes were found in MeHg-treated embryos as well as autophagic vacuoles similar to mitophagic profiles. In addition, we observed autophagic vacuoles with amorphous, homogeneous, and electron-dense contents, similar to the autophagy. Our results showed, for the first time, the neurotoxic effect of MeHg on the ultrastructure of the developing spinal cord. Using TEM we demonstrate that changes in the endomembrane system, mitochondrial damage, disturbance in mitochondrial dynamics, and increase in mitophagy were caused by MeHg exposure.
Figures



References
-
- Amin-Zaki L., Elhassani S., Majeed M. A., Clarkson T. W., Doherty R. A., Greenwood M. Intra-uterine methylmercury poisoning in Iraq. Pediatrics. 1974;54(5):587–595. - PubMed
-
- Choi B. H., Lapham L. W., Amin-Zaki L., Saleem T. Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain: a major effect of methylmercury poisoning in utero. Journal of Neuropathology & Experimental Neurology. 1978;37(6):719–733. doi: 10.1097/00005072-197811000-00001. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

