Clinically-Relevant Rapamycin Treatment Regimens Enhance CD8+ Effector Memory T Cell Function In The Skin and Allow their Infiltration into Cutaneous Squamous Cell Carcinoma
- PMID: 30228949
- PMCID: PMC6140608
- DOI: 10.1080/2162402X.2018.1479627
Clinically-Relevant Rapamycin Treatment Regimens Enhance CD8+ Effector Memory T Cell Function In The Skin and Allow their Infiltration into Cutaneous Squamous Cell Carcinoma
Abstract
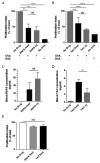
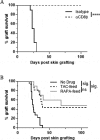
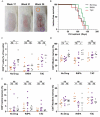
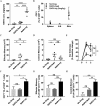
Patients receiving immunosuppressive drugs to prevent organ transplant rejection exhibit a greatly increased risk of developing cutaneous squamous cell carcinoma (SCC). However, not all immunosuppressive drugs confer the same risk. Randomised, controlled trials demonstrate that switching renal transplant recipients receiving calcineurin inhibitor-based therapies to mammalian target of rapamycin (mTOR) inhibitors results in a reduced incidence of de novo SSC formation, and can even result in the regression of pre-existing premalignant lesions. However, the contribution played by residual immune function in this setting is unclear. We examined the hypotheses that mTOR inhibitors promote the enhanced differentiation and function of CD8+ memory T cells in the skin. Here, we demonstrate that the long-term oral administration of rapamycin to achieve clinically-relevant whole blood drug target thresholds, creates a "low rapamycin dose" environment in the skin. While both rapamycin and the calcineurin inhibitor tacrolimus elongated the survival of OVA-expressing skin grafts, and inhibited short-term antigen-specific CD8+ T cell responses, rapamycin but not tacrolimus permitted the statistically significant infiltration of CD8+ effector memory T cells into UV-induced SCC lesions. Furthermore, rapamycin uniquely enhanced the number and function of CD8+ effector and central memory T cells in a model of long-term contact hypersensitivity provided that rapamycin was present during the antigen sensitization phase. Thus, our findings suggest that patients switched to mTOR inhibitor regimens likely experience enhanced CD8+ memory T cell function to new antigen-challenges in their skin, which could contribute to their lower risk of de novo SSC formation and regression of pre-existing premalignant lesions.
Keywords: T cells; memory; skin; transplantation.
Figures



References
-
- Jung JW, Overgaard NH, Burke MT, Isbel N, Frazer IH, Simpson F et al. Does the nature of residual immune function explain the differential risk of non-melanoma skin cancer development in immunosuppressed organ transplant recipients?. International Journal of Cancer. 2016;138:281–292. doi: 10.1002/ijc.29450. - DOI - PubMed
-
- Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513–519. - PubMed
-
- McDonald S, Clayton P, Australia HK, Dialysis NZ, Registry T. 2012. Annual report - 35th Edition. Aust New Zealand Dial Transpl Registry. 2012:48–56. http://www.anzdata.org.au/v1/report_2012.html:.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
