A UK nationwide prospective study of treatment change in MODY: genetic subtype and clinical characteristics predict optimal glycaemic control after discontinuing insulin and metformin
- PMID: 30229274
- PMCID: PMC6223847
- DOI: 10.1007/s00125-018-4728-6
A UK nationwide prospective study of treatment change in MODY: genetic subtype and clinical characteristics predict optimal glycaemic control after discontinuing insulin and metformin
Abstract
Aims/hypothesis: Treatment change following a genetic diagnosis of MODY is frequently indicated, but little is known about the factors predicting future treatment success. We therefore conducted the first prospective study to determine the impact of a genetic diagnosis on individuals with GCK-, HNF1A- or HNF4A-MODY in the UK, and to identify clinical characteristics predicting treatment success (i.e. HbA1c ≤58 mmol/mol [≤7.5%]) with the recommended treatment at 2 years.
Methods: This was an observational, prospective, non-selective study of individuals referred to the Exeter Molecular Genetic Laboratory for genetic testing from December 2010 to December 2012. Individuals from the UK with GCK- or HNF1A/HNF4A-MODY who were not on recommended treatment at the time of genetic diagnosis, and who were diagnosed below the age of 30 years and were currently aged less than 50 years, were eligible to participate.
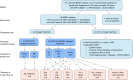
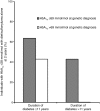
Results: A total of 44 of 58 individuals (75.9%) changed treatment following their genetic diagnosis. Eight individuals diagnosed with GCK-MODY stopped all diabetes medication without experiencing any change in HbA1c (49.5 mmol/mol [6.6%] both before the genetic diagnosis and at a median of 1.25 years' follow-up without treatment, p = 0.88). A total of 36 of 49 individuals (73.5%) diagnosed with HNF1A/HNF4A-MODY changed treatment; however, of the 21 of these individuals who were being managed with diet or sulfonylurea alone at 2 years, only 13 (36.1% of the population that changed treatment) had an HbA1c ≤58 mmol/mol (≤7.5%). These individuals had a shorter diabetes duration (median 4.6 vs 18.1 years), lower HbA1c (58 vs 73 mmol/mol [7.5% vs 8.8%]) and lower BMI (median 24.2 vs 26.0 kg/m2) at the time of genetic diagnosis, compared with individuals (n = 23/36) with an HbA1c >58 mmol/mol (>7.5%) (or <58 mmol/mol [<7.5%] on additional treatment) at the 2 year follow-up. Overall, 64% (7/11) individuals with a diabetes duration of ≤11 years and an HbA1c of ≤69 mmol/mol (≤8.5%) at time of the genetic test achieved good glycaemic control (HbA1c ≤58 mmol/mol [≤7.5%]) with diet or sulfonylurea alone at 2 years, compared with no participants with a diabetes duration of >11 years and an HbA1c of >69 mmol/mol (>8.5%) at the time of genetic diagnosis.
Conclusions/interpretation: In participants with GCK-MODY, treatment cessation was universally successful, with no change in HbA1c at follow-up. In those with HNF1A/HNF4A-MODY, a shorter diabetes duration, lower HbA1c and lower BMI at genetic diagnosis predicted successful treatment with sulfonylurea/diet alone, supporting the need for early genetic diagnosis and treatment change. Our study suggests that, in individuals with HNF1A/HNF4A-MODY with a longer duration of diabetes (>11 years) at time of genetic test, rather than ceasing current treatment, a sulfonylurea should be added to existing therapy, particularly in those who are overweight or obese and have a high HbA1c.
Keywords: Genetic testing; Glucokinase; Hepatocyte nuclear factor 1α; Hepatocyte nuclear factor 4α; Maturity onset diabetes of the young; Sulfonylurea; Treatment change.
Conflict of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

