A kinetic study for the Fenton and photo-Fenton paracetamol degradation in an annular photoreactor
- PMID: 30229488
- PMCID: PMC8298369
- DOI: 10.1007/s11356-018-3098-4
A kinetic study for the Fenton and photo-Fenton paracetamol degradation in an annular photoreactor
Erratum in
-
Correction to: A kinetic study for the Fenton and photo-Fenton paracetamol degradation in an annular photoreactor.Environ Sci Pollut Res Int. 2021 Aug;28(32):44580. doi: 10.1007/s11356-021-15050-7. Environ Sci Pollut Res Int. 2021. PMID: 34196871 Free PMC article. No abstract available.
Abstract
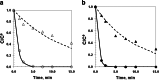
A kinetic model describing Fenton and photo-Fenton degradation of paracetamol (PCT) and consumption of hydrogen peroxide (H2O2) was proposed. A set of Fenton and photo-Fenton experiments (18 runs in total) was performed by fixing the initial concentration of PCT to 40 mg L-1 and varying the initial concentrations of H2O2 and ferrous ion, Fe2+. The experimental set-up was a well-stirred annular photoreactor equipped with an actinic BL TL-DK 36 W/10 1SL lamp. Experimental results highlighted that PCT is no more detected by HPLC analysis within a minimum reaction time of 2.5 and a maximum reaction time of 15.0 min. Besides, a maximum conversion of total organic carbon (TOC) of 68.5% was observed after 75 min of reaction in case of using UV radiation and the highest concentrations of the Fenton reagents. The experimental data were used to fit the kinetic model. The radiation field inside the reactor was taken into account through the local volumetric rate of photon absorption, evaluated by assuming a line source model with spherical and isotropic emission. The kinetic parameters were estimated by using a non-linear least-squares regression procedure and root mean square errors (RMSE) were calculated in order to validate the feasibility of the proposed model. A good agreement between experimental and predicted data was observed and the lowest values of RMSE resulted in 5.84 and 9.59% for PCT and H2O2 normalized concentrations, respectively.
Keywords: AOPs; Annular photoreactor; Design of experiments; Kinetic modeling; LVRPA; Pharmaceuticals.
Figures




References
-
- Alfano OM, Romero RL, Cassano AE. Radiation field modelling in photoreactors-I. Homogeneous media. Chem Eng Sci. 1986;41(3):421–444.
-
- Andreozzi R, Caprio V, Insola A, Marotta R. Advanced oxidation processes (AOP) for water purification and recovery. Catal Today. 1999;53(1):51–59.
-
- Andreozzi R, D’Apuzzo A, Marotta R. A kinetic model for the degradation of benzothiazole by Fe3+-photo-assisted Fenton process in a completely mixed batch reactor. J Hazard Mater B. 2000;80(1–3):241–257. - PubMed
-
- Antunes SC, Freitas R, Figueira E, Gonçalves F, Nunes B. Biochemical effects of acetaminophen in aquatic species: edible clams Venerupis decussata and Venerupis philippinarum. Environ Sci Pollut Res. 2013;20(9):6658–6666. - PubMed
-
- Bossmann SH, Oliveros E, Göb S, Siegwart S, Dahlen EP, PayawanJr L, Straub M, Wörner M, Braun AM. New evidence against hydroxyl radicals as reactive intermediates in the thermal and photochemically enhanced Fenton reactions. J Phys Chem A. 1998;102(28):5542–5550.
MeSH terms
Substances
Grants and funding
- PIC50420150100009LI/Universidad Nacional del Litoral
- PIP-2015 0100093/Consejo Nacional de Investigaciones Científicas y Técnicas
- PICT-2015-2651/Agencia Nacional de Promoción Científica y Tecnológica
- DPI2017-87435-R/Ministerio de Economía, Industria y Competitividad
- BES-2013-065545/Ministerio de Economía y Competitividad
LinkOut - more resources
Full Text Sources
Other Literature Sources

