Novel preclinical murine model of trauma-induced elbow stiffness
- PMID: 30229498
- PMCID: PMC6143496
- DOI: 10.1186/s40634-018-0155-3
Novel preclinical murine model of trauma-induced elbow stiffness
Abstract
Background: Peri-articular injury may result in functional deficits and pain. In particular, post-traumatic elbow stiffness is a debilitating condition, precluding patients from performing activities of daily living. As such, clinicians and basic scientists alike, aim to develop novel therapeutic interventions to prevent and treat elbow stiffness; thereby reducing patient morbidity. Yet, there is a paucity of pre-clinical models of peri-articular stiffness, especially of the upper extremity, necessary to develop and test the efficacy of therapeutics. We set out to develop a pre-clinical murine model of elbow stiffness, resulting from soft tissue injury, with features characteristic of pathology observed in these patients.
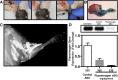
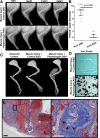
Methods: A soft tissue peri-elbow injury was inflicted in mice using cardiotoxin. Pathologic tissue repair was induced by creating an investigator-imposed deficiency of plasminogen, a protease essential for musculoskeletal tissue repair. Functional testing was conducted through analysis of grip strength and gait. Radiography, microcomputed tomography, and histological analyses were employed to quantify development of heterotopic ossification.
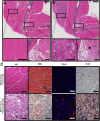
Results: Animals with peri-elbow soft tissues injury in conjunction with an investigator-imposed plasminogen deficiency, developed a significant loss of elbow function measured by grip strength (2.387 ± 0.136 N vs 1.921 ± 0.157 N, ****, p < 0.0001) and gait analysis (35.05 ± 2.775 mm vs 29.87 ± 2.075 mm, ***, p < 0.0002). Additionally, plasminogen deficient animals developed capsule thickening, delayed skeletal muscle repair, fibrosis, chronic inflammation, and heterotopic ossification; all features characteristic of pathology observed in patients with trauma-induced elbow stiffness.
Conclusion: A soft tissue injury to the peri-elbow soft tissue with a concomitant deficiency in plasminogen, instigates elbow stiffness and pathologic features similar to those observed in humans. This pre-clinical model is valuable for translational studies designed to investigate the contributions of pathologic features to elbow stiffness or as a high-throughput model for testing therapeutic strategies designed to prevent and treat trauma-induced elbow stiffness.
Keywords: Elbow stiffness; Elbow trauma; Murine model; Plasmin; Plasminogen; Preclinical model.
Conflict of interest statement
Ethics approval
All animal procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee (M1600225) and carried out in strict accordance with the recommendation in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Consent for publication
Not applicable
Competing interests
JGS is a member of the education advisory board at OrthoPediatrics, receives research funding from OrthoPediatrics, and research support from IONIS Pharmaceuticals. JGS and SNML receive research and training support from PXE International. All other authors have declared that no conflict of interest exists.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
The post-traumatic stiff elbow: A review.J Clin Orthop Trauma. 2021 May 19;19:125-131. doi: 10.1016/j.jcot.2021.05.006. eCollection 2021 Aug. J Clin Orthop Trauma. 2021. PMID: 34277339 Free PMC article.
-
Treatment of ectopic ossification about the elbow.Clin Orthop Relat Res. 2000 Jan;(370):65-86. doi: 10.1097/00003086-200001000-00008. Clin Orthop Relat Res. 2000. PMID: 10660703 Review.
-
Development and use of an animal model to study post-traumatic stiffness and contracture of the elbow.J Orthop Res. 2016 Feb;34(2):354-64. doi: 10.1002/jor.22981. Epub 2015 Jul 31. J Orthop Res. 2016. PMID: 26177969
-
Trauma-Induced Nanohydroxyapatite Deposition in Skeletal Muscle is Sufficient to Drive Heterotopic Ossification.Calcif Tissue Int. 2019 Apr;104(4):411-425. doi: 10.1007/s00223-018-0502-5. Epub 2018 Dec 4. Calcif Tissue Int. 2019. PMID: 30515544 Free PMC article.
-
Postburn Contractures of the Elbow and Heterotopic Ossification.Hand Clin. 2017 May;33(2):375-388. doi: 10.1016/j.hcl.2016.12.014. Hand Clin. 2017. PMID: 28363302 Review.
Cited by
-
Maintaining the balance: the critical role of plasmin activity in orthopedic surgery injury response.J Thromb Haemost. 2023 Oct;21(10):2653-2665. doi: 10.1016/j.jtha.2023.08.002. Epub 2023 Aug 8. J Thromb Haemost. 2023. PMID: 37558131 Free PMC article. Review.
-
Preclinical Models of Elbow Injury and Pathology.Ann Jt. 2021 Jan;6:12. doi: 10.21037/aoj.2020.02.09. Epub 2021 Jan 15. Ann Jt. 2021. PMID: 35990575 Free PMC article.
-
The Prognosis of Arthrofibroses: Prevalence, Clinical Shortcomings, and Future Prospects.Trends Pharmacol Sci. 2021 May;42(5):398-415. doi: 10.1016/j.tips.2021.02.007. Epub 2021 Mar 29. Trends Pharmacol Sci. 2021. PMID: 33795150 Free PMC article. Review.
-
Functional Measures of Grip Strength and Gait Remain Altered Long-term in a Rat Model of Post-traumatic Elbow Contracture.J Biomech Eng. 2019 Apr 8;141(7):0710011-8. doi: 10.1115/1.4043433. Online ahead of print. J Biomech Eng. 2019. PMID: 30958506 Free PMC article.
References
-
- Amaro E, et al. Abstract P20: severe injury leads to plasmin consumption below a critical threshold required to heal soft tissue injury. Plastic and Reconstructive Surgery – Global Open. 2017;5:115–116. doi: 10.1097/01.GOX.0000516677.91747.d0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

