Development and content validity of a hemodialysis symptom patient-reported outcome measure
- PMID: 30229532
- PMCID: PMC6339833
- DOI: 10.1007/s11136-018-2000-7
Development and content validity of a hemodialysis symptom patient-reported outcome measure
Abstract
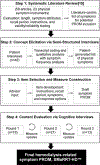
Purpose: To describe the process and preliminary qualitative development of a new symptom-based patient-reported outcome measure (PROM) intended to assess hemodialysis treatment-related physical symptoms.
Methods: Experienced interviewers conducted concept elicitation and cognitive debriefing interviews with individuals receiving in-center hemodialysis in the United States. Concept elicitation interviews involved eliciting spontaneous reports of symptom experiences and probing to further explore and confirm concepts. We used patient-reported concepts to generate a preliminary symptom PROM. We conducted 3 rounds of cognitive debriefing interviews to evaluate symptom relevance, item interpretability, and draft item structure. We iteratively refined the measure based on cognitive interview findings.
Results: Forty-two adults receiving in-center hemodialysis participated in the concept elicitation interviews. A total of 12 symptoms were reported by > 10% of interviewees. We developed a 13-item initial draft instrument for testing in 3 rounds of cognitive interviews with an additional 52 hemodialysis patients. Participant responses and feedback during cognitive interviews led to changes in symptom descriptions, division of the single item "nausea/vomiting" into 2 distinct items, removal of daily activity interference items, addition of instructions, and clarification about the recall period, among other changes.
Conclusions: Symptom Monitoring on Renal Replacement Therapy-Hemodialysis (SMaRRT-HD™) is a 14-item PROM intended for use in hemodialysis patents. SMaRRT-HD™ uses a single treatment recall period and a 5-point Likert scale to assess symptom severity. Qualitative interview data provide evidence of its content validity. SMaRRT-HD™ is undergoing additional testing to assess measurement properties and inform measure scoring.
Keywords: Cognitive debriefing interview; Concept elicitation; Content validity; End-stage kidney disease; Hemodialysis; Patient-reported outcome measure; Symptoms.
Conflict of interest statement
Figures
References
-
- Merkus MP, Jager KJ, Dekker FW, de Haan RJ, Boeschoten EW, Krediet RT: Physical symptoms and quality of life in patients on chronic dialysis: results of The Netherlands Cooperative Study on Adequacy of Dialysis (NECOSAD). Nephrol Dial Transplant 1999, 14(5):1163–1170. - PubMed
-
- Weisbord SD, Fried LF, Arnold RM, Fine MJ, Levenson DJ, Peterson RA, Switzer GE: Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol 2005, 16(8):2487–2494. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical