Pharmacokinetics, Excretion, and Mass Balance of [14 C]-Batefenterol Following a Single Microtracer Intravenous Dose (Concomitant to an Inhaled Dose) or Oral Dose of Batefenterol in Healthy Men
- PMID: 30230263
- PMCID: PMC6282586
- DOI: 10.1002/cpdd.616
Pharmacokinetics, Excretion, and Mass Balance of [14 C]-Batefenterol Following a Single Microtracer Intravenous Dose (Concomitant to an Inhaled Dose) or Oral Dose of Batefenterol in Healthy Men
Erratum in
-
Correction.Clin Pharmacol Drug Dev. 2019 Oct;8(7):971. doi: 10.1002/cpdd.719. Epub 2019 Jul 12. Clin Pharmacol Drug Dev. 2019. PMID: 31589387 Free PMC article. No abstract available.
Abstract
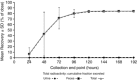
Inhaled batefenterol is an investigational bifunctional molecule for the treatment of chronic obstructive pulmonary disease. The excretion balance and pharmacokinetics of batefenterol using [14 C]-radiolabeled drug administered orally and as intravenous (IV) infusion were assessed. In this 2-period, open-label study, 6 healthy male subjects received a single IV microtracer 1-hour infusion of 4 μg [14 C]-batefenterol concomitant with inhaled nonradiolabeled batefenterol (1200 μg) followed by oral [14 C]-batefenterol (200 μg) in period 2 after a 14-day washout. The primary end points included: the area under the concentration-time curve from time zero to last time of quantifiable concentration (AUC0-t ); maximum observed concentration (Cmax ); and time of occurrence of maximum observed concentration. Following IV administration, the geometric mean AUC0-t of [14 C]-batefenterol was 121.9 pgEq • h/mL; maximum observed concentration and time of occurrence of maximum observed concentration were 92.7 pgEq/mL and 0.8 hours, respectively; absolute oral bioavailability was 0.012%. The mean AUC0-t ratio indicated that [14 C]-batefenterol accounted for 85% of total circulating radioactivity in the plasma initially and declined rapidly following IV administration, but only ∼0.2% of total circulating radioactivity following oral administration. Cumulative mean recovery of total radioactive [14 C]-batefenterol in urine and feces was 6.31% and 77.6%, respectively. Overall, batefenterol exhibited low systemic bioavailability after inhaled and oral administration, and high fecal excretion and low urinary excretion following IV and oral administration.
Trial registration: ClinicalTrials.gov NCT02663089.
Keywords: batefenterol; chiral inversion; microtracer; pharmacokinetics.
© 2018, The Authors. Clinical Pharmacology in Drug Development published by Wiley Periodicals, Inc. on behalf of The American College of Clinical Pharmacology.
Figures




Similar articles
-
Open-Label, Randomized, 6-Way Crossover, Single-Dose Study to Determine the Pharmacokinetics of Batefenterol (GSK961081) and Fluticasone Furoate When Administered Alone or in Combination.Clin Pharmacol Drug Dev. 2016 Sep;5(5):399-407. doi: 10.1002/cpdd.274. Epub 2016 Jun 26. Clin Pharmacol Drug Dev. 2016. PMID: 27170445 Clinical Trial.
-
Revefenacin Absorption, Metabolism, and Excretion in Healthy Subjects and Pharmacological Activity of Its Major Metabolite.Drug Metab Dispos. 2020 Dec;48(12):1312-1320. doi: 10.1124/dmd.120.000103. Epub 2020 Sep 25. Drug Metab Dispos. 2020. PMID: 32978223 Clinical Trial.
-
Pharmacokinetics and absorption, distribution, metabolism and excretion profiling of tanimilast following an intravenous 14C-microtracer coadministered with an inhaled dose in healthy male individuals.Drug Metab Dispos. 2025 Jan;53(1):100009. doi: 10.1124/dmd.124.001895. Epub 2024 Nov 22. Drug Metab Dispos. 2025. PMID: 39884821
-
Mini-Review: Comprehensive Drug Disposition Knowledge Generated in the Modern Human Radiolabeled ADME Study.CPT Pharmacometrics Syst Pharmacol. 2020 Aug;9(8):428-434. doi: 10.1002/psp4.12540. Epub 2020 Jul 31. CPT Pharmacometrics Syst Pharmacol. 2020. PMID: 32562380 Free PMC article. Review.
-
Human absorption, distribution, metabolism, and excretion studies: Conventional or microtracer?Drug Metab Dispos. 2025 May;53(5):100067. doi: 10.1016/j.dmd.2025.100067. Epub 2025 Mar 19. Drug Metab Dispos. 2025. PMID: 40198958 Review.
Cited by
-
Long-Acting Muscarinic Antagonists Under Investigational to Treat Chronic Obstructive Pulmonary Disease.J Exp Pharmacol. 2020 Dec 8;12:559-574. doi: 10.2147/JEP.S259330. eCollection 2020. J Exp Pharmacol. 2020. PMID: 33324119 Free PMC article. Review.
-
Investigation of the human metabolism and disposition of the prolyl hydrolase inhibitor daprodustat using IV microtracer with Entero-Test bile string.Pharmacol Res Perspect. 2023 Dec;11(6):e1145. doi: 10.1002/prp2.1145. Pharmacol Res Perspect. 2023. PMID: 37885335 Free PMC article. Clinical Trial.
References
-
- Global Initiative for Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global strategy for diagnosis, management and prevention of COPD. http://www.goldcopd.org. Published 2017. Accessed July 2017.
-
- Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. - PubMed
-
- Cazzola M, Noschese P, Salzillo A, De Giglio C, D'Amato G, Matera MG. Bronchodilator response to formoterol after regular tiotropium or to tiotropium after regular formoterol in COPD patients. Respir Med. 2005;99(5):524–528. - PubMed
-
- Cazzola M, Molimard M. The scientific rationale for combining long‐acting beta2‐agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010;23(4):257–267. - PubMed
-
- Hegde SS, Hughes AD, Chen Y, et al. Pharmacologic characterization of GSK‐961081 (TD‐5959), a first‐in‐class inhaled bifunctional bronchodilator possessing muscarinic receptor antagonist and beta2‐adrenoceptor agonist properties. J Pharmacol Exp Ther. 2014;351(1):190–199. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

